Calculate E cell for each of the following concentration cells: (a) Cu(s) Cu+ (aq, 0.0010 mol-L-)||Cu+ (aq,
Question:
Calculate Ecell for each of the following concentration cells: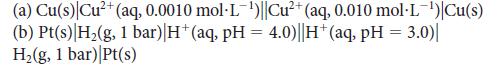
Transcribed Image Text:
(a) Cu(s) Cu²+ (aq, 0.0010 mol-L-¹)||Cu²+ (aq, 0.010 mol-L-¹')|Cu(s) (b) Pt(s) |H₂(g, 1 bar)|H*(aq, pH = 4.0)||H+ (aq, pH = 3.0)| H₂(g, 1 bar) Pt(s)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
a 0...View the full answer

Answered By

Benish Ahmad
I'm a professional software engineer. I'm lectutrer at GCUF and I have 3 years of teaching experience. I'm looking forward to getting mostly computer science work including:
Programming fundamentals
Object oriented programming
Data structures
object oriented design and analysis
Database system
Computer networks
Discrete mathematics
Web application
I am expert in different computer languages such as C++, java, JavaScript, Sql, CSS, Python and C#. I'm also have excellent knowledge of essay writing and research. I have worked in other Freelancing website such as Fiverr and Upwork. Now I have finally decided to join the SolutionInn platform to continue with my explicit work of helping dear clients and students to achieve their academic dreams. I deliver plagiarism free work and exceptional projects on time. I am capable of working under high pressure.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Looking at a simple box of name brand cereal, approxmately, what percentage of the retail price has gone towards the cos of advertising? O 90% O 30% 50% O 10%
-
Calculate the unknown concentration of the ion in each of the following cells: 2+ (a) Pb(s) Pb+ (aq, ?)||Pb+ (aq, 0.10 mol-L-) Pb(s), Ecell = +0.083 V. (b) Pt(s)| Fe+ (aq, 0.10 mol-L-), Fe+ (aq, 1.0...
-
PURPOSE This task helps you prepare for some group work in class. We opened the course by recognizing that we have gut reactions to business messages. Now that we've covered a good deal of theory...
-
Tubby Toys estimates that its new line of rubber ducks will generate sales of $7 million, operating costs of $4 million, and a depreciation expense of $1 million. If the tax rate is 35%, what is the...
-
Exercises in compound interest, no income taxes. To be sure that you understand how to use the tables in Appendix B at the end of this book, solve the following exercises. Ignore income tax...
-
The titanium content in an aircraft-grade alloy is an important determinant of strength. A sample of 20 test coupons reveals the following titanium content (in percent): 8.32, 8.05, 8.93, 8.65, 8.25,...
-
Calculate the values for leverage, standardized residual, and Cooks distance for the 11th hiker who had hiked for 10 hours and traveled 23 kilometers. Show that, while it is neither an outlier nor of...
-
A researcher wishes to compare two hotels on the following attributes: Convenience of location Friendly personnel Value for money a. Design a Likert scale to accomplish this task. b. Design a...
-
Mountain Dental Services is a specialized dental practice whose only service is filling cavities. Mountain has recorded the following for the past nine months: Month January February March April June...
-
Hotel DelRay is located at the heart of the city of Brussels, in Belgium. Brussels is a major hub for international politics, a home for several international organizations and diplomats, and a...
-
Calculate the pH and pOH of (a) A solution that is 0.50 m NaHSO 4 (aq) and 0.25 m Na 2 SO 4 (aq); (b) A solution that is 0.50 m NaHSO 4 (aq) and 0.10 m Na 2 SO 4 (aq); (c) A solution that is 0.50 m...
-
Suppose that 1.436 g of impure sodium hydroxide is dissolved in 300. mL of aqueous solution and that 25.00 mL of this solution is titrated to the stoichiometric point with 34.20 mL of 0.0695 m...
-
A gas stream of 70 mol % methane and 30 mol % carbon dioxide is available 15 bar and 200 K. To decrease the concentration of carbon dioxide in the methane, the gas stream will be flashed by flowing...
-
A storeroom is used to organize items stored in it on N shelves. Shelves are numbered from 0 to N-1. The K-th shelf is dedicated to items of only one type, denoted by a positive integer A[K]....
-
CASES CASE 10.1 Money in Motion Jake Nguyen runs a nervous hand through his once finely combed hair. He loosens his once perfectly knotted silk tie. And he rubs his sweaty hands across his once...
-
(3.8) Axiom, Definition of false false = true (3.9) Axiom, Distributivity of over : (pq) p=q
-
The board of directors of Unilever has been impressed by the presentation you did, and they further instructed you to conduct a more insightful investigation about the Sri Lankan market. They have...
-
The sample space listing the eight simple events that are possible when a couple has three children is {bbb, bbg, bgb, ogg, gbb, gbg, ggb, ggg}. After identifying the sample space for a couple having...
-
Consider the distribution of Trypanosoma lengths shown by the density curve in Exercise 3.4.3. Consider the length of an individual trypanosome chosen at random from the population. Find (a)...
-
An educational researcher devised a wooden toy assembly project to test learning in 6-year-olds. The time in seconds to assemble the project was noted, and the toy was disassembled out of the childs...
-
Draw all constitutional isomers with molecular formula C 3 H 9 N, and provide a name for each isomer.
-
Rank this group of compounds in order of increasing boiling point. -NH2 -N-
-
Identify whether each of the following compounds is expected to be water soluble: (a) (b) (c) -NH2 -NH2
-
Need help filling out these tax forms. Not sure how to do 1040 page 2 or schedule 3. I think I have schedule 1 right but need help with the itemized deductions for 1040 page 1 Required information...
-
Question:What should Airbus and Boing have learned from IBERIA case? What changed in the industry when Boing decided to develop Dreamliner in 2003?( Read the following case and ppt) Airline Route...
-
Which of the following needs to be always assessed when you are evaluating the literature you have obtained for your research? O All of the above O Sufficiency Value O Relevance Several approaches...

Study smarter with the SolutionInn App


