Calculate the potential of a cell constructed with two nickel electrodes. The electrolyte in one compartment is
Question:
Calculate the potential of a cell constructed with two nickel electrodes. The electrolyte in one compartment is 1.0 m Ni(NO3)2(aq).
In the other compartment, NaOH has been added to a Ni(NO3)2 solution until the pH = 11.0 at 298 K. See Table 6I.1.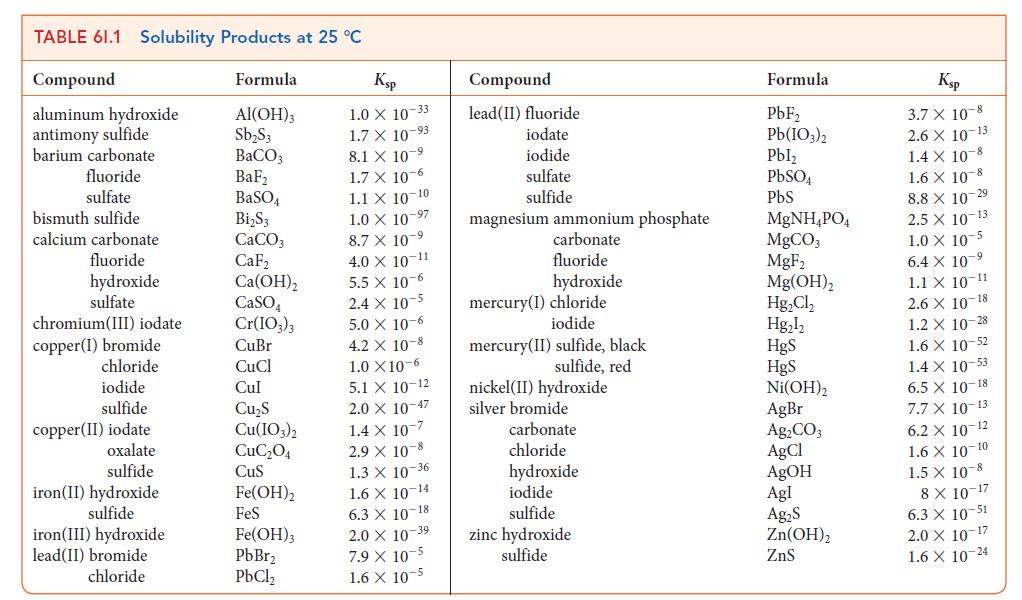
Transcribed Image Text:
TABLE 61.1 Solubility Products at 25 °C Compound aluminum hydroxide antimony sulfide barium carbonate fluoride sulfate bismuth sulfide calcium carbonate fluoride hydroxide sulfate chromium (III) iodate copper (1) bromide chloride iodide sulfide copper(II) iodate oxalate sulfide iron(II) hydroxide sulfide iron(III) hydroxide lead(II) bromide chloride Formula Al(OH)3 Sb₂S3 BaCO3 BaF₂ BaSO4 Bi₂S3 CaCO3 CaF₂ Ca(OH)₂ CaSO4 Cr(IO3)3 CuBr CuCl Cul Cu₂S Cu(IO3)2 CuC₂04 CuS Fe(OH)2 FeS Fe(OH)3 PbBr₂ PbCl₂ Ksp 1.0 X 10-33 1.7 X 10-93 8.1 X 107 1.7 x 10-6 1.1 X 10-10 1.0 X 10-97 8.7 X 10-9 4.0 X 10-11 5.5 x 10-6 2.4 x 10-5 5.0 x 10-6 4.2 X 10-8 1.0 X10-6 5.1 X 10-12 2.0 X 10-47 1.4 x 10-7 2.9 X 10-8 1.3 X 10-36 1.6 X 10-14 6.3 X 10-18 2.0 X 10 39 7.9 X 10-5 1.6 X 10-5 Compound lead(II) fluoride iodate iodide sulfate sulfide magnesium ammonium phosphate carbonate fluoride hydroxide mercury(I) chloride iodide mercury (II) sulfide, black sulfide, red nickel (II) hydroxide silver bromide carbonate chloride hydroxide iodide sulfide zinc hydroxide sulfide Formula PbF₂ Pb(103)2 Pbl₂ PbSO4 PbS MgNH₂PO4 MgCO3 MgF₂ Mg(OH)₂ Hg₂Cl₂ Hg₂l₂ HgS HgS Ni(OH)2 AgBr Ag₂CO3 AgCl AgOH Agl Ag₂S Zn(OH)₂ ZnS Ksp 3.7 X 10-8 2.6 X 10-13 1.4 X 10-8 1.6 X 10 8 8.8 X 10-29 2.5 X 10-13 1.0 X 10-5 6.4 X 10 ⁹ 1.1 X 10-11 2.6 X 10-18 1.2 X 10-28 1.6 X 10-52 1.4 x 10-53 6.5 X 10-18 7.7 X 10-13 6.2 X 10-12 1.6 X 10-10 1.5 X 10-8 8 X 10-17 6.3 X 10-51 2.0 X 10-17 1.6 X 10-24
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)

Answered By

ANDREW KIPRUTO
Academic Writing Expert
I have over 7 years of research and application experience. I am trained and licensed to provide expertise in IT information, computer sciences related topics and other units like chemistry, Business, law, biology, biochemistry, and genetics. I'm a network and IT admin with +8 years of experience in all kind of environments.
I can help you in the following areas:
Networking
- Ethernet, Wireless Airmax and 802.11, fiber networks on GPON/GEPON and WDM
- Protocols and IP Services: VLANs, LACP, ACLs, VPNs, OSPF, BGP, RADIUS, PPPoE, DNS, Proxies, SNMP
- Vendors: MikroTik, Ubiquiti, Cisco, Juniper, HP, Dell, DrayTek, SMC, Zyxel, Furukawa Electric, and many more
- Monitoring Systems: PRTG, Zabbix, Whatsup Gold, TheDude, RRDtoo
Always available for new projects! Contact me for any inquiries
4.30+
1+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
A cell was constructed with two lead electrodes. The electrolyte in one compartment is 1.0 m Pb(NO 3 ) 2 (aq). In the other compartment, NaI has been added to a Pb(NO 3 ) 2 solution until a yellow...
-
Calculate the potential of a copper electrode immersed in (a) 0.0380 M Cu(NO3)2. (b) 0.0650 M in NaCl and saturated with CuCl. (c) 0.0350 M in NaOH and saturated with Cu(OH)2. (d) 0.0375 M in...
-
Find the probability | (p)| 2d3 p of the particular momentum p for the ground-state hydrogen atom. (This is a nice exercise in three-dimensional Fourier transforms. To perform the angular...
-
If you are running at top speed toward a source of sound at 1000 Hz, estimate the frequency of the sound that you hear. Suppose that you can recognize a change in frequency of 3%. Can you use your...
-
The Pinkerton Publishing Company is considering two mutually exclusive expansion plans. Plan A calls for the expenditure of $50 million on a large-scale, integrated plant which will provide an...
-
6. Suppose that I is a nonempty, open interval and f : 1-+ Rm is differentiable on I. If f(I) ~ aBr(O) for some fixed r > 0, prove that f(t) is orthogonal to f'(t) for all tEl.
-
Demo Consulting is a consulting firm owned and operated by Jesse Flatt. The following end-of period spreadsheet was prepared for the year ended August 31, 20Y9: Based on the preceding spreadsheet,...
-
Jointly owned real property is reported on A) form 706 Schedule A B) form 706 Sched E C) form 706 Sch F D) form 706 Sch J
-
TourneSol Canada, Ltd. is a producer of high quality sunflower oil. The company buys raw sunflower seeds directly from large agricultural companies, and refines the seeds into sunflower oil that it...
-
The pK a of HIO(aq), hypoiodous acid, is 10.64 and that of HIO 3 (aq), iodic acid, is 0.77. Account for the difference in strength.
-
You find a bottle of a pure silver halide that could be AgCl or AgI. Develop a simple chemical test that would allow you to distinguish which compound was in the bottle.
-
Redo Problem 3.69 if a 1-in.-diameter nozzle is placed at the end of the tube. Problem 3.69 Water is siphoned from the tank shown in Fig. P3.69. Determine the flowrate from the tank and the pressure...
-
Answer the following problems with solution: Use the following information for the next two questions: The statement of financial position of the partnership of A and B as of December 31, 20x1 is...
-
Complete the following budgets 1 Production Budget Planned Sales Desired Ending Inventory of Finished Goods (roundup to the next unit) Total Needed Less: Beginning Inventory Total Production {7.01}
-
Solution needs urgently. Question 1 (5 points) In times of prosperity (with high incomes and employment), governments at all levels have resources for high cost infrastructure such as roads,...
-
Write a program (called assignment-1.cxx)-- (40 points) Write down the C++ program based on the following tasks. Creates an Array (1D array) and randomly assign values. Show the array with assigned...
-
on 17:03 Sat 11 May < 00 194843... 19871 II B itsSUNPI is live! + Hi mates im Alive chating and all the fun stuf that we do O COME & GET ME! -- Sunpi FE now Untitled... HYPE RESULTS T Potential...
-
Consider a 32-bit microprocessor, with a 16-bit external data bus, driven by an 8-MHz input clock. Assume that this microprocessor has a bus cycle whose minimum duration equals four input clock...
-
Inexhaustible collections of ONPOs are not required to be capitalized or depreciated, if certain criteria are met. Why is this so, and what accounting and reporting recognition, if any, is required...
-
Several reactions and their standard reaction enthalpies at 298.15 K are given here: The standard enthalpies of combustion of graphite and C 2 H 2 (g) are 393.51 and 1299.58 kJ mol 1 , respectively....
-
For each type of bond below, determine the direction of the expected dipole moment. a) C O b) C Mg c) C N d) C Li e) C Cl f) C H g) O H h) N H
-
Given the following heat capacity data, calculate ÎH o f of CO 2 (g) at 525 K. C(graphite) co:(g) 0:(g) Substance CP.m/J mol-K-1 8.52 28.8 37.1
-
Simpson Ltd is a small IT company, which has 2 million shares outstanding and a share price of $20 per share. The management of Simpson plans to increase debt and suggests it will generate $3 million...
-
The following are the information of Chun Equipment Company for Year 2 . ( Hint: Some of the items will not appear on either statement, and ending retained earnings must be calculated. ) Salaries...
-
Alta Ski Company's inventory records contained the following information regarding its latest ski model. The company uses a periodic inventory system. Beginning inventory, January 1, 2018 1,250 units...

Study smarter with the SolutionInn App


