Calculate the reaction enthalpy for the formation of anhydrous aluminum bromide, 2 Al(s) + 3 Br 2
Question:
Calculate the reaction enthalpy for the formation of anhydrous aluminum bromide, 2 Al(s) + 3 Br2(l) → 2 AlBr3(s), from the following data: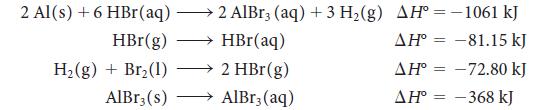
Transcribed Image Text:
2 Al(s) + 6 HBr(aq) - HBr(g) H₂(g) + Br₂ (1) AlBr3 (s) 2 AlBr3 (aq) + 3 H₂(g) AH-1061 kJ HBr(aq) AH° -81.15 kJ 2 HBr(g) AH° -72.80 kJ AlBr3(aq) AH-368 kJ = =
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)
We need to manipulate and combine these two equations to obtain the desired reaction equation ...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Calculate the reaction enthalpy for the synthesis of hydrogen bromide gas, H 2 (g) + Br 2 (l) 2 HBr(g), from the following data: NH3(g) + HBr (g) N(g) + 3 H(g) N(g) + 4 H(g) + Br (1) NH,Br(s)...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
Barium metal is produced by the reaction of aluminum metal with barium oxide. From the standard reaction enthalpies, calculate the reaction enthalpy for the production of metallic barium in the...
-
QUESTION 17 The moment of a force about a pivot point is; a. The force multiplied by the perpendicular distance fro the pivot point to the direction of the force b. the distance from the pivot to the...
-
Bowyer Manufacturing Company has the following production data for selected months. Compute the physical units for eachmonth. Ending Work in Process % Complete as to Conversion Cost Beginning Work in...
-
Use the formula in the indicated entry of the Table of Integrals on Reference Pages 6 10 to evaluate the integral. Sxa |x entry 87 arcsin(x) dx;
-
3. Pla, a partner in the Bri Partnership, has a 30 percent participation in partnership profits and losses. Plas capital account had a net decrease of $60,000 during the calendar year 2016. During...
-
Affordable Lawn Care, Inc., provides lawn-mowing services to both commercial and residential customers. The company performs adjusting entries on a monthly basis, whereas closing entries are prepared...
-
P2-1B Pedriani Company uses a job order cost system and applies overhead to production Prepare entries in a job order on the basis of direct labor hours. On January 1, 2017, Job No. 25 was the only...
-
Use standard enthalpies of formation from Appendix 2A to calculate the standard reaction enthalpy for each of the following reactions: (a) The final stage in the production of nitric acid: (b) The...
-
Calculate the standard entropy of vaporization of water at 85C, given that its standard entropy of vaporization at 100.C is 109.0 J K 1 mol 1 and the molar heat capacities at constant pressure of...
-
Lucas Air Service, a sole proprietorship, provides charter flights on weekdays only. Earl Lucas, the owner, is thinking of offering flying lessons on weekends. He has always pro- tected his weekends...
-
Based on the reading,How to make sure your next product or service launch drives growth (click the underlined link),what stands out to you as the most important factor in a differentiated launch...
-
OM in the News has previously looked at the Waffle House Index, used to measure the damage from hurricanes. The index made the news again for Hurricane Ian. According to the Boston Globe, 40 Waffle...
-
Mixture of persuasive and negative formal I am Elizabeth grinderFirst part email Next part setting up the meeting Final part memo The reader is Robert * do not come off accusatory*** Project TWO:...
-
Anyone who has sampled today's social media offerings has probably experienced this situation: You find a few fascinating blogs, a few interesting people to follow on Twitter, a couple of podcast...
-
a. Begin with a converging lens of focal length f. Place an illuminated object a distance p, in front of the lens. For all positive values of p;: 1. calculate and sketch a graph of the location of...
-
A Drosophila embryo dies during early embryogenesis due to a recessive maternal effect allele called bicoid. The wild-type allele is designated bicoid*. What are the genotypes and phenotypes of the...
-
On January 2, 20X3, Sheldon Bass, a professional engineer, moved from Calgary to Edmonton to commence employment with Acco Ltd., a large public corporation. Because of his new employment contract,...
-
Oxidation of an aldehyde yields a carboxylic acid: Draw the structures for the products of the following oxidation reactions. a. b. c. [ox] propanal 2,3-dimethylpentanal ox] 3-ethylbenzaldehyde>
-
Three different organic compounds have the formula C3H8O. Only two of these isomers react with KMnO4 (a strong oxidizing agent). What are the names of the products when these isomers react with...
-
Give an example reaction that would yield the following products as major organic products. For oxidation reactions, just write oxidation over the arrow and dont worry about the actual reagent. a....
-
Break-Even Sales and Sales to Realize Income from Operations For the current year ending October 31, Yentling Company expects fixed costs of $537,600, a unit variable cost of $50, and a unit selling...
-
You buy a stock for $35 per share. One year later you receive a dividend of $3.50 per share and sell the stock for $30 per share. What is your total rate of return on this investment? What is your...
-
Filippucci Company used a budgeted indirect-cost rate for its manufacturing operations, the amount allocated ($200,000) is different from the actual amount incurred ($225,000). Ending balances in the...

Study smarter with the SolutionInn App


