Calculate the standard Gibbs free energy for each of the following reactions: 2 HI(g), K = 54
Question:
Calculate the standard Gibbs free energy for each of the following reactions: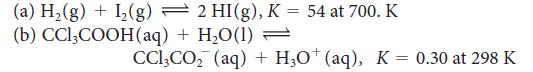
Transcribed Image Text:
2 HI(g), K = 54 at 700. K (a) H₂(g) + 1₂(g) (b) CCl3COOH (aq) + H₂O(1) = CCl₂CO₂ (aq) + H3O+ (aq), K = 0.30 at 298 K
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
The standard Gibbs free energy change AG for a reaction at a given temperature ...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
The oxidation of SO 2 to SO 3 is one of the reactions involved in the formation of acid rain. If you want to predict the spontaneous direction of the reaction for a specific mixture of the gases, you...
-
(a) Using values in Appendix 2A, calculate the standard Gibbs free energy for the vaporization of water at 25.0C, 100.0C, and 150.0C. (b) What should the value at 100.0C be? (c) Why is there a...
-
Calculate the standard enthalpy, entropy, and Gibbs free energy for each of the following reactions at 298 K by using data in Appendix 2A. For each case, confirm that the value obtained from the...
-
1. Insurance Act, RSBC 1996 c226 Read Parts 1 and 2 of this statute and describe any changes to the standard commonlaw rules for contracts that you notice. 2. KP Pacific Holdings Ltd. and Churchland...
-
Reconsider the state-transition diagram in Figure. Describe, in words or with a diagram, a similar state-transition diagram for a system with three processes and a single resource type with two units...
-
Prepare the Annual Reconciliation of Employer Wage Tax for Philadelphia, using the blank. For lines 1, 3, and 4, use gross wages and salaries per general ledger less exempt wages paid to Russell...
-
potential regression-related problems of which you should be aware
-
(a) What specific recommendations would you give the Johnsons for selecting checking and savings accounts that will enable them to effectively use the first and second tools of monetary asset...
-
You could purchase a zero coupon bond that has a maturity value of $25000 and earns a current market rate of 3 percent. If the bond matures in 10 years and we assume semiannual compounding, what is...
-
A conducting bar is connected via flexible leads to a pair of rails in a magnetic field B = 6 cos 10t ax mWb/m2 as in figure. If the z-axis is the equilibrium position of the bar and its velocity is...
-
What is the molality of acetone, C 3 H 6 O, in an aqueous solution for which the mole fraction of acetone is 0.112?
-
Permanganate ions are powerful oxidizing agents used in water treatment facilities to remove metals, such as iron, and toxic and malodorous chemicals, such as H 2 S. If you are using permanganate...
-
Why do organisations need managers? Give your own views of the importance of management in the hospitality industry.
-
1.For Zenith Co. the Processing Division sells a computer module to the company's Assembly Division, which assembles the finished product.The Microprocessor Division is currently working at capacity....
-
I. The company "EVERYTHING FRESH EXCEPT THE CHICKEN" buys birds to process and distribute them for human consumption. From the process of these birds 3 products "breasts, thighs and wings" are...
-
What is the decimal value of this 8-bit two's complement number? 1000 0000
-
Direct Disk Drive Company operates a computer disk manufacturing plant. Direct materials are added at the end of the process. The following data were for June2017: Work in process, beginning...
-
You are the director of health information management at an acute care hospital. The hospital's radiology manager has come to you because an employee mistakenly included protected health information...
-
Write the digits 1,2,3,4 in order on an index card. Bring this card to a busy place (e.g., dining hall, library, university union) and ask at least 30 people to look at the card and select one of the...
-
In order to get an idea on current buying trends, a real estate agent collects data on 10 recent house sales in the area. Specifically, she notes the number of bedrooms in each house as follows: a....
-
Why does the energy of a rotating molecule depend on l , but not on m l ?
-
Are the real functions listed in Equations (18.62) and (18.63) eigenfunctions of l z ? Justify your answer.
-
Spatial quantization was discussed in Section 18.8. Suppose that we have a gas consisting of atoms, each of which has a nonzero angular momentum. Are all of their angular momentum vectors aligned?
-
Questien It Calraluta bae neark yoe cen atforal to berren
-
In calculating the net present value of a proposed project, the cash flows of the project should include a.) amortization of goodwill b.) interest expenses paid to bondholders c.) extra working...
-
If Yolanda's insurance company cancels her fire insurance policy after 204 days, how much of the $682.00 annual premium will she receive as a refund (in $)? (Round you answer to the nearest cent.) $

Study smarter with the SolutionInn App


