Permanganate ions are powerful oxidizing agents used in water treatment facilities to remove metals, such as iron,
Question:
Permanganate ions are powerful oxidizing agents used in water treatment facilities to remove metals, such as iron, and toxic and malodorous chemicals, such as H2S. If you are using permanganate solutions, you need to know the exact concentrations. Titration with oxalic acid is commonly used to determine the concentration of solutions of MnO4 –, so you need to know the balanced redox equation. Permanganate ions, MnO4 –, oxidize oxalic acid, H2C2O4, in acidic aqueous solution. The skeletal equation (with states added) for the reaction is![]()
Write the balanced net ionic equation for this reaction.
PLAN To balance the equation, work through the procedure for an acidic solution set out in Toolbox 6K.1.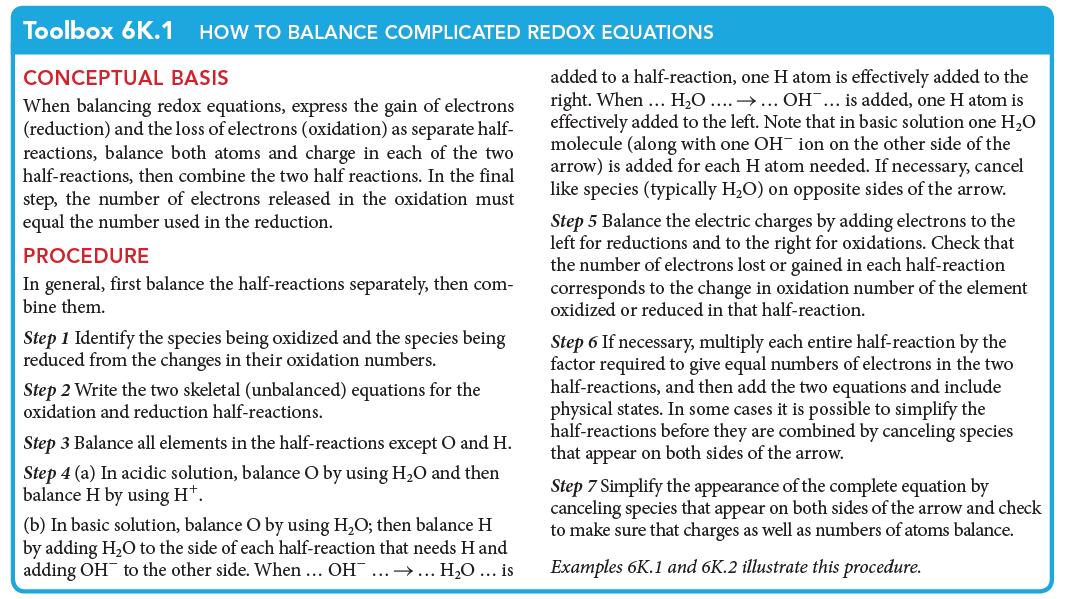
Step by Step Answer:

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman





