You are working in an analytical laboratory and have been asked to use the permanganate solution you
Question:
You are working in an analytical laboratory and have been asked to use the permanganate solution you prepared in Example 6K.1 to determine the concentration of bromide ions in a sample of groundwater sent to you by an environmental agency. The products of the reaction between bromide ions and permanganate ions, MnO4–, in basic aqueous solution are solid manganese(IV) oxide, MnO2, and bromate ions, BrO3–. Balance the net ionic equation for the reaction.
PLAN Work through the procedure for a basic solution set out in Toolbox 6K.1.
Example 6K.1
Permanganate ions are powerful oxidizing agents used in water treatment facilities to remove metals, such as iron, and toxic and malodorous chemicals, such as H2S. If you are using permanganate solutions, you need to know the exact concentrations. Titration with oxalic acid is commonly used to determine the concentration of solutions of MnO4 –, so you need to know the balanced redox equation. Permanganate ions, MnO4 –, oxidize oxalic acid, H2C2O4, in acidic aqueous solution. The skeletal equation (with states added) for the reaction is![]()
Write the balanced net ionic equation for this reaction.
PLAN To balance the equation, work through the procedure for an acidic solution set out in Toolbox 6K.1.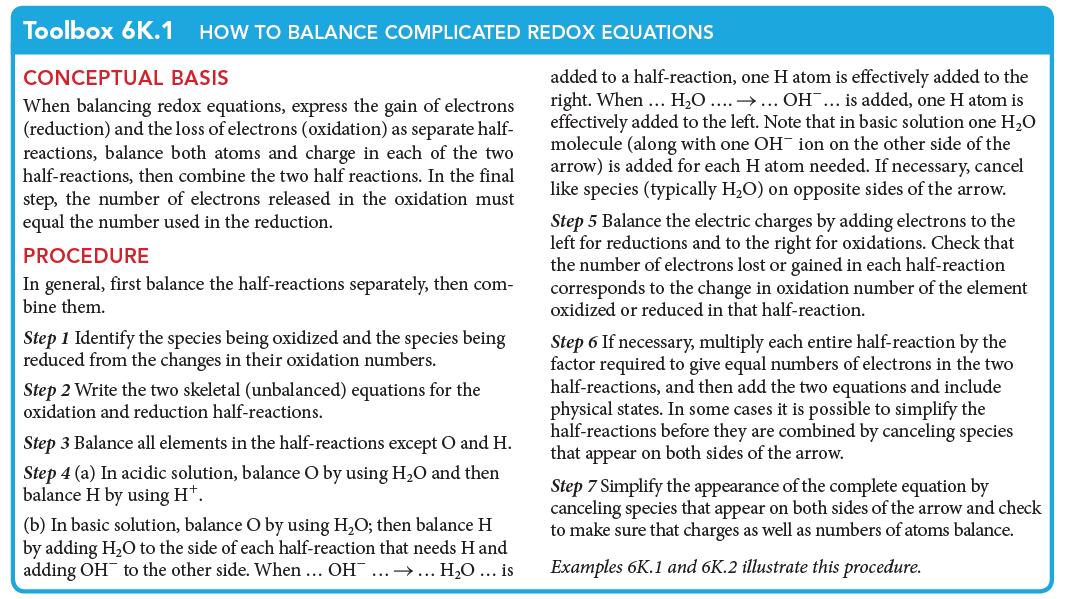
Step by Step Answer:

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman





