One of the largest uses of electricity is in the production of aluminum by electrolysis of its
Question:
One of the largest uses of electricity is in the production of aluminum by electrolysis of its oxide dissolved in molten cryolite (Na3AlF6). As an engineer, you might need to predict how much aluminum can be produced by this process. Find the mass of aluminum that can be produced in 1.00 day in an electrolytic cell operating continuously at 1.00 * 105 A. The cryolite does not react.
ANTICIPATE In this industrial-scale process, with a high current acting for a long time, you should expect to generate many kilograms of aluminum.
PLAN Use the first procedure in Toolbox 6O.1.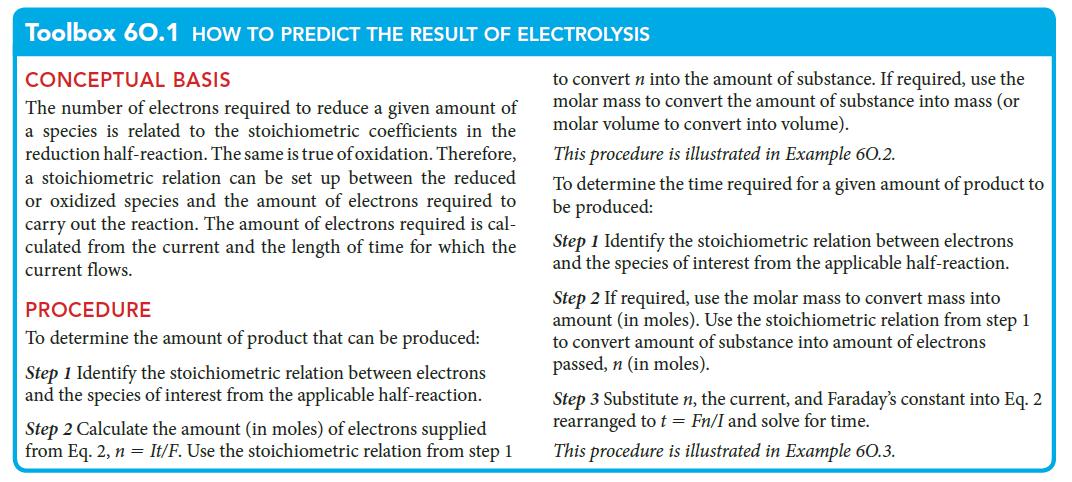
Transcribed Image Text:
Toolbox 60.1 HOW TO PREDICT THE RESULT OF ELECTROLYSIS CONCEPTUAL BASIS The number of electrons required to reduce a given amount of a species is related to the stoichiometric coefficients in the reduction half-reaction. The same is true of oxidation. Therefore, a stoichiometric relation can be set up between the reduced or oxidized species and the amount of electrons required to carry out the reaction. The amount of electrons required is cal- culated from the current and the length of time for which the current flows. PROCEDURE To determine the amount of product that can be produced: Step 1 Identify the stoichiometric relation between electrons and the species of interest from the applicable half-reaction. Step 2 Calculate the amount (in moles) of electrons supplied from Eq. 2, n = It/F. Use the stoichiometric relation from step 1 to convert n into the amount of substance. If required, use the molar mass to convert the amount of substance into mass (or molar volume to convert into volume). This procedure is illustrated in Example 60.2. To determine the time required for a given amount of product to be produced: Step 1 Identify the stoichiometric relation between electrons and the species of interest from the applicable half-reaction. Step 2 If required, use the molar mass to convert mass into amount (in moles). Use the stoichiometric relation from step 1 to convert amount of substance into amount of electrons passed, n (in moles). Step 3 Substitute n, the current, and Faraday's constant into Eq. 2 rearranged to t = Fn/I and solve for time. This procedure is illustrated in Example 60.3.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
Step 1 Write the halfreaction for the reduction and find the amount of electrons required to reduce ...View the full answer

Answered By

Shehar bano
I have collective experience of more than 7 years in education. my area of specialization includes economics, business, marketing and accounting. During my study period I remained engaged with a business school as a visiting faculty member and did a lot of business research. I am also tutoring and mentoring number of international students and professionals online for the last 7 years.
5.00+
4+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
The Crazy Eddie fraud may appear smaller and gentler than the massive billion-dollar frauds exposed in recent times, such as Bernie Madoffs Ponzi scheme, frauds in the subprime mortgage market, the...
-
1. How strong are the competitive forces confronting J. Crew in the market for specialty retail? Do a [Michael Porter] five-forces analysis to support your answer. (see chapter 3 in the textfor...
-
Read below and provide an executive summary key issues GAP, like most retailers, has been going through a tough time in recent years. There have been several factors that contributed to the suffering...
-
1. Explain the Theories of leadership 2. Research -In modern times give 1 example as to which business leader in the hospitality industry has made a mark and why.?
-
Cost allocation responsibility accounting ethics In 2019, only 806,840 Deliman meals were produced and sold to the hospitals. Smith suspects that hospital controllers had systematically inflated...
-
A transformer is made up of a 1200-turn primary coil and an open-circuited 75-turn secondary coil wound around a closed core of cross-sectional area 42 cm2. The core material can be considered to...
-
List the outliers. What do all these outliers have in common? For Orlando Palmeiro, explain why he is an outlier.
-
Using the following data from the comparative balance sheet of Dotte Company, illustrate horizontalanalysis. December 31, 2014 $520,000 $840,000 $2,500,000 December 31, 2013 Accounts receivable...
-
T-Mobile currently has a customer retention rate of 63 %. Can someone please help me with this? Calculate the expected customer lifetime for an average T-Mobile customer. Calculate your answer to one...
-
Peta is married, filing joint with two other dependents, is paid $17.25 per hour, and receives commission on sales. No commission is paid out until sales exceed $60,000. Once the minimum sales have...
-
Calculate the pH of 6.55 * 10 7 m HClO 4 (aq).
-
You are working in an analytical laboratory and have been asked to use the permanganate solution you prepared in Example 6K.1 to determine the concentration of bromide ions in a sample of groundwater...
-
The insurance company where you work is planning to raise all premiums for health-care coverage. Your boss has asked you to read a draft of her letter to customers announcing the new, higher rates....
-
Swenson Company produced 300 units in year one and sold 260 units in that year. In year two, it produced 260 units and sold 300 units. Total fixed overhead was the same in years one and two. Under...
-
c) Determine the maximum rotational speed such that the fluid will not spill over the container. (and: = 2gh/R) [2 marks] d) The container in Figure 4 now contains coffee (p~1000) which is 7cm deep...
-
FICO credit scores: x = 564,= 743,= 72 (Round your answer to 3 decimal places.) what does z equal
-
Q3: In the section illustrated in Figure (1) the surface 1-4-7 is insulated. The convection heat transfer coefficient at surface 1-2-3 is 28 W/m. 'C. The thermal conductivity of the solid material is...
-
25 of 27 > This test: 96 point(s) possible This question: 3 point(s) possible Submit test Identical twins come from a single egg that split into two embryos, and fraternal twins are from separate...
-
The rowan (Sorbus aucuparia) is a tree that grows in a wide range of altitudes. To study how the tree adapts to its varying habitats, researchers collected twigs with attached buds from 12 trees...
-
Establish identity. cos( + k) = (-1)k cos , k any integer
-
A bowling ball has a weight of 12 lb and the length of the lane is approximately 60. feet. Treat the ball in the lane as a one-dimensional box. What quantum number corresponds to a velocity of 7.5...
-
For a particle in a two-dimensional box, the total energy eigenfunctions are a. Obtain an expression for E nx , n y in terms of n x , n y , a, and b by substituting this wave function into the two...
-
Consider the contour plots of Problem P15.17. a. What are the most likely area or areas Îx Îy to find the particle for each of the eigenfunctions of HË depicted in plots af? b. For...
-
A family has a $117,443, 25-year mortgage at 5.4% compounded monthly. (A) Find the monthly payment and the total interest paid. (B) Suppose the family decides to add an extra $100 to its mortgage...
-
Comparing the actual and planned cost of a consulting engagement completed by an engineering firm such as Allied Engineering.
-
What is the NPV of a project that costs $34,000 today and is expected to generate annual cash inflows of $11,000 for the next 7 years, followed by a final inflow of $14,000 in year 8. Cost of capital...

Study smarter with the SolutionInn App


