Calculate the standard Gibbs free energy of each of the following reactions: (a) I(g) 2 I(g), K
Question:
Calculate the standard Gibbs free energy of each of the following reactions: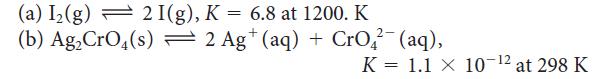
Transcribed Image Text:
(a) I₂(g) 2 I(g), K = 6.8 at 1200. K (b) Ag₂ CrO4(s) = 2 Ag+ (aq) + CrO₂²(aq), K 1.1 X 10-12 at 298 K
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 85% (7 reviews)
a AG ...View the full answer

Answered By

Ishrat Khan
Previously, I have worked as an accounting scholar at acemyhomework, and have been tutoring busines students in various subjects, mostly accounting. More specifically I'm very knowledgeable in accounting subjects for college and university level. I have done master in commerce specialising in accounting and finance as well as other business subjects.
5.00+
140+ Reviews
437+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
The oxidation of SO 2 to SO 3 is one of the reactions involved in the formation of acid rain. If you want to predict the spontaneous direction of the reaction for a specific mixture of the gases, you...
-
(a) Calculate the standard Gibbs free energies of formation of the halogen atoms X (g) at 1000. K from data available in Table 5G.2. (b) Show how these data correlate with the XX bond strength by...
-
Ammonia survives indefinitely in air. An agronomist studying how long ammonia survives in soil might need to know whether it survives because its oxidation is not spontaneous under ordinary...
-
In Exercises 3538, evaluate C F dr. F(x, y, z) = xi + yj + zk C: r(t) = 2 cos ti + 2 sin tj + tk, 0t 2
-
Find the current in each resistor of the circuit shown in Figure. 12 V
-
A 745i BMW car can brake to a stop in a distance of 121 ft. from a speed of 60.0 mi/h. To brake to a stop from a speed of 80.0 mi/h requires a stopping distance of 211 ft. What is the average braking...
-
(Multiple- and Single-step Income, Retained Earnings) The following account balances were included in the trial balance of J.R. Reid Corporation at June 30, 2004. The Retained Earnings account had a...
-
A U.S.-based MNC has a subsidiary in France (local currency, euro, ¬). The balance sheet and income statement of the subsidiary follow. On December 31, 2112, the exchange rate is US$1.20/¬....
-
The Wildhorse Inn is a restaurant that specializes in southwestern style meals in a moderate price range. Chris Anderson, the manager of Wildhorse, has determined that during the past two years the...
-
The proposed rates were not in the range the CEO expected given the pricing analysis. The CEO has asked the pricing actuary to verify the total projected loss cost excluding potential large storm...
-
The equilibrium constant Kc for the reaction N 2 (g) + O 2 (g) 2 NO (g) at 1200C is 1.00 * 10 5 . Calculate the equilibrium molar concentrations of NO, N 2 , and O 2 in a reaction vessel of volume...
-
Distinguish between an emulsion and a gel. Give at least one example of each.
-
What is the purpose of having multiple levels of page or segment tables rather than a single table for looking up address translations? What are the disadvantages, if any, of this scheme?
-
In Exercises 29 and 30, find the probabilities and indicate when the "5% guideline for cumbersome calculations" is used. 29. Medical Helicopters In a study of helicopter usage and patient survival,...
-
Introduction to Internetworking Project 1: Ctrl-Alt-Del Inc. INTRODUCTION You have accepted a contract to participate in the design of the network infrastructure of a company called Ctrl-Alt-Del Inc....
-
Construct Arguments Tell whether each statement is always true, sometimes true, or never true. Explain. a. An integer is a whole number. b. A natural number is a rational number. c. An irrational...
-
Please answer the following Questions : 1. Who are the competitors for Whole Foods? 2. Do you consider traditional supermarkets to be competitors for natural and organic supermarkets? 3. How would...
-
LNC Corp is trying to determine the effect of its inventory turnover ratio and DSO on its cash conversion. Credit sales in 2016 is $101,000, cost of goods sold will be 70% of sales and it earned a...
-
Calculate the magnitude and the location of the resultant pressure force on the annular gate shown in Figure P2.5.8. im Hub Hub m Figure P2.5.8
-
What is a lobbyist in US? How did this term emerge?
-
When (R)-3-bromo-2, 3-dimethylpentane is treated with sodium hydroxide, four different alkenes are formed. Draw all four products, and rank them in terms of stability. Which product do you expect to...
-
When 3-bromo-2, 4-dimethylpentane is treated with sodium hydroxide, only one alkene is formed. Draw the product and explain why this reaction has only one regiochemical outcome.
-
Predict the major product for each of the following E2 reactions: a. b. c. d. NaOH Br NaOH Br
-
Discuss how debt restructuring, settlement, or modification works. Discuss the journal entries for debtor and creditor
-
Could CNL be a viable business? If so, under what conditions and what level of production (and, since production is directly related to production workers, employees)? All information provided for...
-
the internal operation rulea of cooperation are known As ?

Study smarter with the SolutionInn App


