(a) Calculate the standard Gibbs free energies of formation of the halogen atoms X (g) at 1000....
Question:
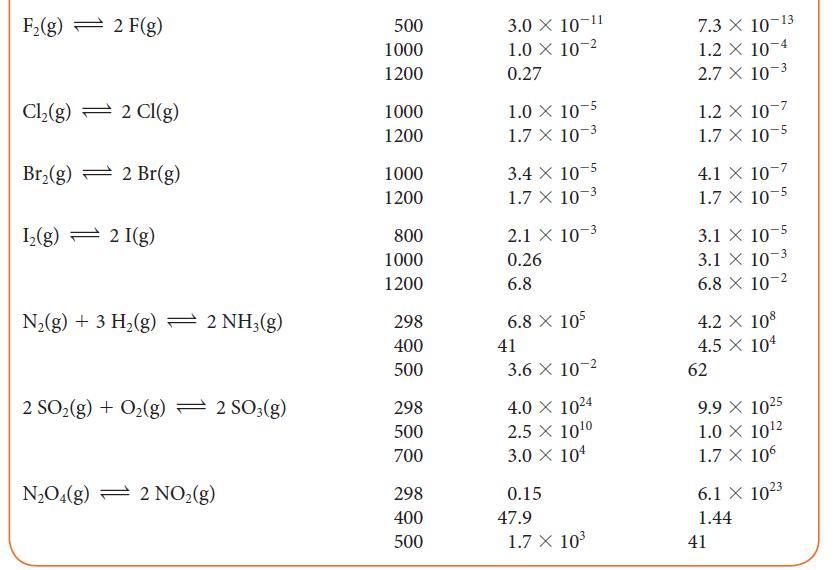
(a) Calculate the standard Gibbs free energies of formation of the halogen atoms X(g) at 1000. K from data available in Table 5G.2.
(b) Show how these data correlate with the X—X bond strength by plotting the standard Gibbs free energy of formation of the atoms against the bond dissociation energy and atomic number. Rationalize any trends you observe.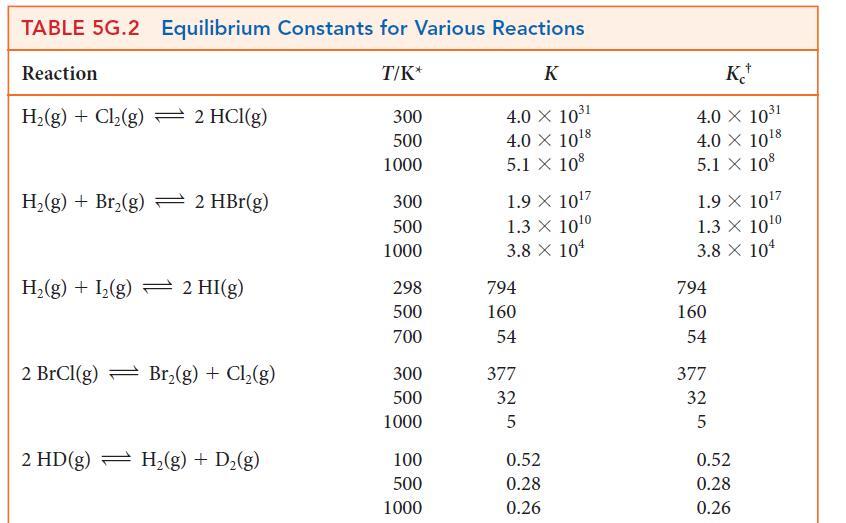
Transcribed Image Text:
TABLE 5G.2 Equilibrium Constants for Various Reactions T/K* Reaction H₂(g) + Cl₂(g) 2 HCl(g) H₂(g) + Br₂(g) 2 Hbr(g) H₂(g) + 1₂(g) = 2 HI(g) 2 BrCl(g) Br₂(g) + Cl₂(g) 2 HD(g) H₂(g) + D₂(g) 300 500 1000 300 500 1000 298 500 700 300 500 1000 100 500 1000 K 4.0 × 10³1 4.0 × 10¹8 5.1 X 108 1.9 X 10¹7 1.3 X 10¹⁰ 3.8 X 104 794 160 54 377 32 5 0.52 0.28 0.26 K 4.0 × 10³1 4.0 × 10¹8 5.1 X 108 1.9 X 10¹7 1.3 × 10¹0 3.8 X 104 794 160 54 377 32 5 0.52 0.28 0.26
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
a Halogen Fluorine Chlorine Bromine Iodine Bond Dissociation ...View the full answer

Answered By

Asim farooq
I have done MS finance and expertise in the field of Accounting, finance, cost accounting, security analysis and portfolio management and management, MS office is at my fingertips, I want my client to take advantage of my practical knowledge. I have been mentoring my client on a freelancer website from last two years, Currently I am working in Telecom company as a financial analyst and before that working as an accountant with Pepsi for one year. I also join a nonprofit organization as a finance assistant to my job duties are making payment to client after tax calculation, I have started my professional career from teaching I was teaching to a master's level student for two years in the evening.
My Expert Service
Financial accounting, Financial management, Cost accounting, Human resource management, Business communication and report writing. Financial accounting : • Journal entries • Financial statements including balance sheet, Profit & Loss account, Cash flow statement • Adjustment entries • Ratio analysis • Accounting concepts • Single entry accounting • Double entry accounting • Bills of exchange • Bank reconciliation statements Cost accounting : • Budgeting • Job order costing • Process costing • Cost of goods sold Financial management : • Capital budgeting • Net Present Value (NPV) • Internal Rate of Return (IRR) • Payback period • Discounted cash flows • Financial analysis • Capital assets pricing model • Simple interest, Compound interest & annuities
4.40+
65+ Reviews
86+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Use the standard Gibbs free energies of formation in Appendix 2A to calculate G for each of the following reactions at 25 C. Comment on the spontaneity of each reaction under standard conditions at...
-
Use the standard Gibbs free energies of formation in Appendix 2A to calculate G for each of the following reactions at 25 C. Comment on the spontaneity of each reaction under standard conditions at...
-
In Exercises 1126, determine whether each equation defines y as a function of x. x + y = 16
-
Two batteries with emf E1 and E2 and internal resistances r1 and r2 are connected in parallel. Prove that if a resistor is connected in parallel with this combination, the optimal load resistance...
-
How does cost of goods sold differ for merchandising versus manufacturing companies?
-
Journal entries related to the income statement. Toyota Motor Company (Toyota), the Japanese car manufacturer, reported Sales of Products of 22,670 billion for the year ended March 31, 2007. The Cost...
-
I % = ( 6 . 6 1 ) P V = - 1 0 3 5 . 5 0 P h t = 1 0 0 0 * 0 . 0 6 7 5 = 6 7 . 0 F V = lon. e . 6 . 1 7 percent e New York Jets have paid a constant annual dividend of $ 1 . 7 7 a share for the past 1...
-
John Campbell, an employee of Manhattan Construction Company, claims to have injured his back as a result of a fall while repairing the roof at one of the Eastview apartment buildings. He filed a...
-
Consider the reaction 2 NO 2 (g) 2 NO(g) + O 2 (g). If the initial molar concentration of NO 2 (g) is 0.030 mol L 1 , and c is the equilibrium molar concentration of O 2 (g) in moles per liter,...
-
In an experiment, 0.100 mol SO 3 was introduced into a flask of volume 2.00 L and the reaction 2 SO 2 (g) + O 2 (g) 2 SO 3 (g) was allowed to come to equilibrium at 700 K. (a) Using information in...
-
Accounting and decision making. Equilibrium, Inc., uses a cost of capital rate of I 2 percent in making investment decisions. It currently is considering two mutually exclusive projects, each...
-
Coaching for Performance Develop a strategy for how you will approach the coaching session with the employee, including what you plan to discuss and any questions you may have when you debrief....
-
For the following exercises, find the derivatives of the given functions: 1. y=x-secx+1 2. y = 3 cscx+ 5 3. f(x) = x cotx 4. f(x) = secx I 5. y=
-
1. why does Amazon use ERP system? How does ERP system work for Amazon? what are the benefit and drawbacks of using ERP for Amazon? 2. what are 5 industry best practices across Finance,...
-
A rigid vessel contains afuel gasconsisting of a methane (CH4) and ethane (C2H6) mixture. The pressure in the vessel is found to be 0.30 bar.Air is added to the vessel until the total pressure...
-
Do you agree with this discussion post? My article discusses decision-making tools in Project Management (PM). "In research and development (R&D), project management (PM) decision-making tools are...
-
Water flows through a 4 -cm, wrought iron, horizontal pipeline from point A to point B. The pipeline is 50 meters long and contains a fully open gate valve and two elbows (RID = 4). If the pressure...
-
Copy and complete the statement. 3800 m ? km =
-
Draw the structure of cis-3,4-diethylcyclobutene. (a) Conrotatory ring opening produces only one product. Draw the product and determine whether its formation is best achieved under thermal...
-
Identify whether the product obtained from each of the following reactions is a meso compound or a pair of enantiomers: (a) Irradiation of (2E,4Z,6Z )-4,5-dimethyl-2,4,6-octatriene with UV light (b)...
-
For each of the following reactions, use brackets and two numbers to identify the type of sigmatropic rearrangement taking place: (a) (b) Heat TH. Heat
-
Which of the following accounts will not be closed during the closing process? a. Accounts Recelvable b. Wages Expense c. Fees Earned d. Rent Expense
-
Clarkson Lumber Company After a rapid growth in its business during recent years, the Clarkson Lumber Company, in the spring of 1996, anticipated a further substantial increase in sales. Despite good...
-
How do external factors such as changing consumer preferences affect the retail industry?"

Study smarter with the SolutionInn App


