Consider the reaction 2 NO 2 (g) 2 NO(g) + O 2 (g). If the initial
Question:
Consider the reaction 2 NO2(g) ⇌ 2 NO(g) + O2(g). If the initial molar concentration of NO2(g) is 0.030 mol · L–1, and c is the equilibrium molar concentration of O2(g) in moles per liter, which of the following expressions is the correct equilibrium relation?![]()
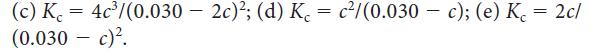
Transcribed Image Text:
(a) K c³; (b) Kc 2c²/(0.030 2c)²;
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)

Answered By

Rishabh Ojha
During my undergraduate i used to participate as TA (Teaching Assistant) in several electronics and computers subject. I'm passionate about learning Computer Science as my bachelors are in Electronics but i learnt most of the Computer Science subjects on my own which Machine Learning also. At Present, i'm a working professional pursuing my career as a Machine Learning Engineer and i want to help others learn during my free hours, that's all the motivation behind giving tuition. To be frank i have no prior experience of tutoring but i have solved problems on opensource platforms like StackOverflow and github. ~Thanks
4.90+
3+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
The reaction 2 NO2 2 NO + O2 has the rate constant. k = 0.63 M-1s-1. Based on the units for k, is the reaction first or second order in NO2? If the initial concentration of NO2 is 0.100M, how would...
-
Consider the reaction 2 CO2 2 CO + O2 obtained after heating 1 kmol CO2 to 3000 K. Find the equilibrium constant from the shift in Gibbs function and verify its value with the entry in Table A.11....
-
What is the molarity of a solution containing 25.5 g KBr dissolved in enough water to make 1.75 L of solution? SORT You are given the mass of KBr and the volume of a solution and asked to find its...
-
The functions in Exercises 1128 are all one-to-one. For each function, a. Find an equation for f -1 (x), the inverse function. b. Verify that your equation is correct by showing that f( f -1 (x)) = x...
-
Capacitors C1 and C2 are connected in parallel by a resistor and two switches as shown in Figure. Capacitor C1 is initially charged to a voltage V0, and capacitor C2 is uncharged. The switches S are...
-
For 6 teams that competed in a competition, their scores are shown below. Which is true of the teams after performing an ANOVA single factor analysis on them? Use a confidence level of 95%. The 6...
-
A manufacturing statement (a) computes cost of goods manufactured for the period, (b) computes cost of goods sold for the period, or (c) reports operating expenses incurred for the period.
-
Consider the following model: a. Provide an interpretation of the coefficient of x1. b. Is the interpretation provided in part a true regardless of the value of x2? Explain. c. Now consider the model...
-
Hello! Can you please post the excel workings through which you got these solutions? Especially the ones with formulas in detailed step by step manner. It will be helpful for students to learn this...
-
Clovis Company recently issued $500,000 (face value) bonds to finance a new construction project. The company's chief accountant prepared the following bond amortization schedule: Required: 1....
-
In an experiment, 0.020 mol NO 2 was introduced into a flask of volume 1.00 L and the reaction 2 NO 2 (g) N 2 O 4 (g) was allowed to come to equilibrium at 298 K. (a) Using information in Table...
-
(a) Calculate the standard Gibbs free energies of formation of the halogen atoms X (g) at 1000. K from data available in Table 5G.2. (b) Show how these data correlate with the XX bond strength by...
-
A plant can manufacture 50 tennis rackets per day for a total daily cost of $3,855 and 60 tennis rackets per day for a total daily cost of $4,245. (A) Assuming that daily cost and production are...
-
The answer above is NOT correct. The value of (2x + 1)(x + x)dx is
-
Review the resource on organizational theory. Explore the various theories and select one to use for this Discussion. Consider the strengths and limitations of the chosen theory. Compose an analysis...
-
How do the locations of Australian department store Myer affect the ability of the other factors of the operating model canvas (suppliers, organization, processes, and information/management systems)...
-
Critical Reading Review: The Exclusion of Latinos from American Media and History Books Read the article. After reading the article, answer the following questions: 1. What purpose do you think the...
-
1. How does the proposed market segment of residential contracts differ from Smith Electric's current market segment? 2.What does a SWOT analysis tell us about Smith Electric's ability to enter a...
-
Water flows from a storage tank through a horizontal, 6-inch pipe (cast iron) and discharges water (20oC) into the atmosphere at the end. The pipe is 500 feet long, contains two I-ft-radius bench and...
-
A line l passes through the points with coordinates (0, 5) and (6, 7). a. Find the gradient of the line. b. Find an equation of the line in the form ax + by + c = 0.
-
Consider the following [4+4] cycloaddition process. Would you expect this process to occur through a thermal or photochemical pathway? Justify your answer with MO theory. +
-
Predict the major product(s) for each of the following thermal electrocyclic reactions: (a) (b) (c) Heat Heat
-
Predict the product for each of the following reactions: (a) (b) (c) Light Light
-
Your company BMG Inc. has to liquidate some equipment that is being replaced. The originally cost of the equipment is $120,000. The firm has deprecated 65% of the original cost. The salvage value of...
-
1. What are the steps that the company has to do in time of merger transaction? And What are the obstacle that may lead to merger failure? 2.What are the Exceptions to not to consolidate the...
-
Problem 12-22 Net Present Value Analysis [LO12-2] The Sweetwater Candy Company would like to buy a new machine that would automatically "dip" chocolates. The dipping operation currently is done...

Study smarter with the SolutionInn App


