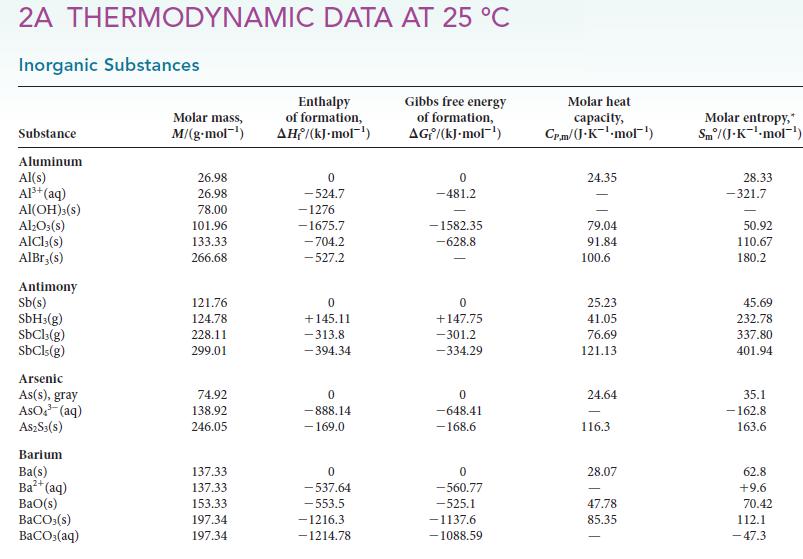
Use the standard Gibbs free energies of formation in Appendix 2A to calculate G for each of
Question:
Use the standard Gibbs free energies of formation in Appendix 2A to calculate ΔG° for each of the following reactions at 25 °C. Comment on the spontaneity of each reaction under standard conditions at 25 °C.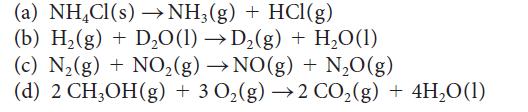
Transcribed Image Text:
(a) NHCl(s) NH(g) + HCl(g) (b) H(g) + DO(l) D(g) + HO(1) (c) N(g) + NO (g) NO(g) + NO(g) (d) 2 CHOH(g) + 3 0(g) 2 CO(g) + 4HO(1)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (5 reviews)
Use the relationship AG AG products AG reactants r a AGAGNH3 g AG HCl gAG NH4Cl s 1645 kJ mol9530 ...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Use the standard Gibbs free energies of formation in Appendix 2A to calculate G for each of the following reactions at 25 C. Comment on the spontaneity of each reaction under standard conditions at...
-
Ammonia survives indefinitely in air. An agronomist studying how long ammonia survives in soil might need to know whether it survives because its oxidation is not spontaneous under ordinary...
-
Use data in Table 4H.1 or Appendix 2A to calculate the standard reaction entropy for each of the following reactions at 25C. For each reaction, interpret the sign and magnitude of the reaction...
-
Consider the exchange rate between South Korea and Costa Rica. Typically, exchange rates vary over time, sometimes quite dramatically. The scenarios present various changes that may affect the...
-
For the cylindrical capacitor of Problem 1.6c, evaluate the variational upper bound of Problem 1.17b with the naive trial function, 1() = (b )/(b a). Compare the variational result with the exact...
-
For the partial solid of revolution in Prob. B/25, determine the mass moment of inertia about the x-axis. x = Problem B/25
-
What are the inputs to a shareholder level discounted cash flow analysis?
-
Selected accounts and related amounts for Drapery Land Co. for the fiscal year ended July 31, 2010, are presented in Problem 6-1B. Instructions 1. Prepare a single-step income statement in the format...
-
Which of the following statements is true of inside-sales personnel? O a. Their primary role is to assist in design and specification processes and provide follow-up technical services. b. They...
-
The following picture shows a molecular visualization of a system undergoing a spontaneous change. Account for the spontaneity of the process in terms of the entropy changes in the system and the...
-
Estimate the molar heat capacity (at constant volume) of sulfur dioxide gas. In addition to translational and rotational motion, there is vibrational motion. Each vibrational degree of freedom...
-
Evaluate the iterated integral. KS (x - 2y) dx dy
-
2. Getting ready for Logarithms and Calculus! a. Fill in the chart and graph the function (I advise practicing on your scientific calculator and desmos. X f(x) = Inx 0 0.5 1 e 10...
-
JoJo Co. had the following balances and information for October. Beg. finished goods inventory = $30 Beg. work in process inventory = $5 Beg. raw materials inventory = $15 End. finished goods...
-
Subway sales have been declining since 2014. In the US, Subway has closed a number of stores due to over-expansion, outdated operations, and uninspiring menus. In Canada, Subway took a different...
-
Harvey Auto Parts purchased a new crane on September 1 for $35,000, paying $10,000 cash and signing a 7%, 12-month note for the remaining balance, interest to be paid at maturity. The crane is...
-
e4(k+1) Find the sum of the series. k = 1 8
-
The following equations are for nuclear reactions that are known to occur in the explosion of an atomic bomb. Identify X. 140 235 144
-
As you rewrite these sentences, replace the cliches and buzzwords with plain language (if you don't recognize any of these terms, you can find definitions online): a. Being a jack-of-all-trades, Dave...
-
Give the structure for each of the following aromatic hydrocarbons. a. o-ethyltoluene b. p -di- tert -butylbenzene c. m -diethylbenzene d. 1-phenyl-2-butene
-
Name each of the following alkenes or alkynes.
-
Name each of the following cyclic alkanes and indicate the formula of the compound.
-
Fig 1. Rolling a 4 on a D4 A four sided die (D4), shaped like a pyramid (or tetrahedron), has 4 flat surfaces opposite four corner points. A number (1, 2, 3, or 4) appears close to the edge of each...
-
I just need help with question #4 please! Thank you! Windsor Manufacturing uses MRP to schedule its production. Below is the Bill of Material (BOM) for Product A. The quantity needed of the part...
-
(25) Suppose that we have an economy consisting of two farmers, Cornelius and Wheaton, who unsurprisingly farm corn c and wheat w, respectively. Assume that both farmers produce their crop of choice...

Study smarter with the SolutionInn App


