Question: Use data in Table 4H.1 or Appendix 2A to calculate the standard reaction entropy for each of the following reactions at 25C. For each reaction,
Use data in Table 4H.1 or Appendix 2A to calculate the standard reaction entropy for each of the following reactions at 25°C. For each reaction, interpret the sign and magnitude of the reaction entropy.
Table 4H.1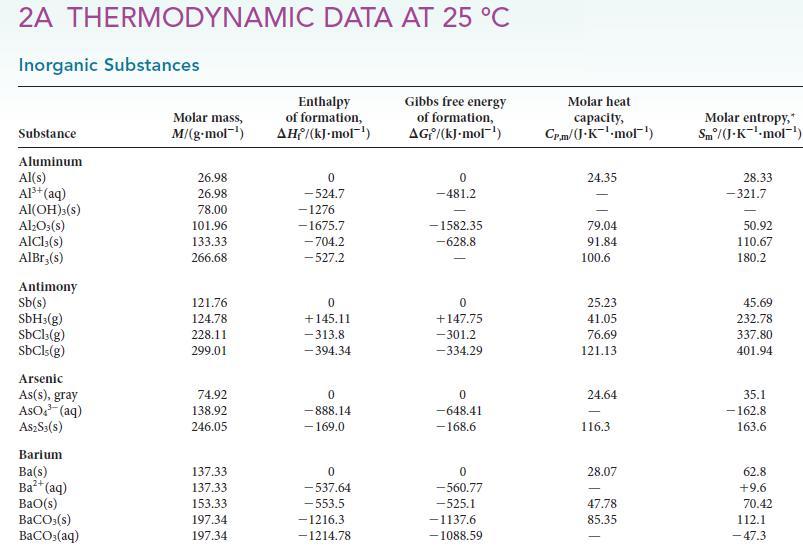
(a) The formation of 1.00 mol H2O(l) from the elements in their most stable state at 298 K and 1 bar.
(b) The oxidation of 1.00 mol CO(g) to carbon dioxide.
(c) The decomposition of 1.00 mol calcite, CaCO3(s), to carbon dioxide gas and solid calcium oxide.
(d) The decomposition of potassium chlorate: 4 KClO3(s) → 3 KClO4(s) + KCl(s).
2A THERMODYNAMIC DATA AT 25 C Inorganic Substances Substance Aluminum Al(s) Al+ (aq) Al(OH)3(s) AlO3(s) AICI, (s) AlBr,(s) Antimony Sb(s) SbH3(g) SbCl3(g) SbCls (g) Arsenic As(s), gray AsO (aq) A$2S3(S) Barium Ba(s) Ba+ (aq) BaO(s) BaCO3(s) BaCO3(aq) Molar mass, M/(g.mol-) 26.98 26.98 78.00 101.96 133.33 266.68 121.76 124.78 228.11 299.01 74.92 138.92 246.05 137.33 137.33 153.33 197.34 197.34 Enthalpy of formation, AH/(kJ-mol-) 0 -524.7 -1276 -1675.7 -704.2 -527.2 0 +145.11 -313.8 -394.34 0 -888.14 - 169.0 0 -537.64 -553.5 -1216.3 - 1214.78 Gibbs free energy of formation, AG/(kJ.mol-) 0 -481.2 -1582.35 -628.8 0 +147.75 -301.2 -334.29 0 -648.41 -168.6 0 -560.77 -525.1 -1137.6 -1088.59 Molar heat capacity, Cr.m/(J.K-mol). 24.35 79.04 91.84 100.6 25.23 41.05 76.69 121.13 24.64 116.3 28.07 47.78 85.35 Molar entropy,* Sm/(J-K-mol-) 28.33 -321.7 - 50.92 110.67 180.2 45.69 232.78 337.80 401.94 35.1 - 162.8 163.6 62.8 +9.6 70.42 112.1 -47.3
Step by Step Solution
3.48 Rating (168 Votes )
There are 3 Steps involved in it
a 16334 JKmol the entropy change is negative because the number of moles of ga... View full answer

Get step-by-step solutions from verified subject matter experts


