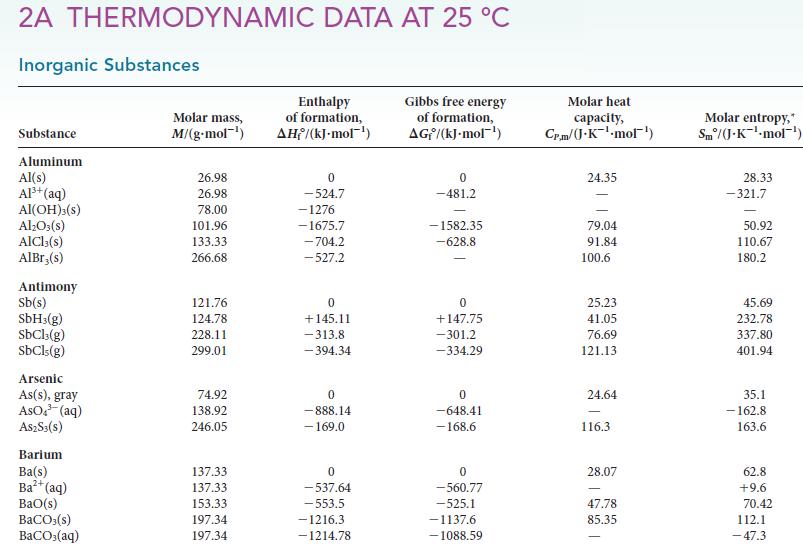
Use standard enthalpies of formation from Appendix 2A to calculate the standard reaction enthalpy for each of
Question:
Use standard enthalpies of formation from Appendix 2A to calculate the standard reaction enthalpy for each of the following reactions:
(a) The removal of hydrogen sulfide from natural gas:![]()
(b) The oxidation of ammonia:![]()
(c) The formation of phosphorous acid:![]()
Transcribed Image Text:
2 HS(g) + SO (g) - 3 S(s) + 2 HO (1)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
a AH3AHSs2AHHO12AHHSgAHSOg 3 mol0 kJ mol2 mol28583 kJ mol 2 m...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Use standard enthalpies of formation from Appendix 2A to calculate the standard reaction enthalpy for each of the following reactions: (a) The final stage in the production of nitric acid: (b) The...
-
Use data in Table 4H.1 or Appendix 2A to calculate the standard reaction entropy for each of the following reactions at 25C. For each reaction, interpret the sign and magnitude of the reaction...
-
The information in Table 4D.2 must be determined from experimental data, but because some reactions cannot be carried out directly, chemists who compile these types of tables commonly use enthalpies...
-
47GP: Chapter: CH0 CH1 CH2 CH3 CH4 CH5 CH6 CH7 CH8 CH9 CH10 CH11 CH12 CH13 CH14 CH15 CH16 CH17 CH18 CH19 CH20 CH21 CH22 CH23 CH24 CH25 CH26 CH27 CH28 CH29 CH30 Problem: 1CQ 1MCP 1P 2CQ 2MCP 2P 3CQ...
-
In Montego Company, total material costs are $32,000, and total conversion costs are $54,000. Equivalent units of production are materials 10,000 and conversion costs 12,000. Compute the unit costs...
-
Which of the following integrals are improper? Why? a. 0 sec x dx b. c. d. '4 dx Jo x - 5
-
E 16-17 Partnership income allocation and new partner investmentVarious situations 1. Cob, Inc., a partner in TLC Partnership, assigns its partnership interest to Ben, who is not made a partner....
-
For each of the following situations, determine the necessary adjustments. 1. A firm purchased a three-year insurance policy for $12,600 on July 1, 2016. The $12,600 was debited to the Prepaid...
-
Assume that you are asked to explain how premiums for a life insurance policy are calculated. Based on the infor- mation in the following table, answer these questions: a. Compute the net single...
-
Calculate the standard enthalpy of formation of dinitrogen pentoxide from the following data: and from the standard enthalpy of formation of nitric oxide, NO. 2 NO(g) + O2(g) + O2(g) 4 NO2(g) 2...
-
Calculate the reaction enthalpy for the formation of anhydrous aluminum bromide, 2 Al(s) + 3 Br 2 (l) 2 AlBr 3 (s), from the following data: 2 Al(s) + 6 HBr(aq) - HBr(g) H(g) + Br (1) AlBr3 (s) 2...
-
Briefly describe the subject of each of the following letter rulings. State the type [PLR, FSA, SCA, etc.] of each letter ruling. a. 201903017 b. 200130045 c. 201449010 d. 200245051
-
You will be creating a Performance Improvement Plan to address an employee in the attached case study (see below). This is a scenario you may encounter in your future HR profession, so this...
-
For this prompt, consider your academic goals, including (but not limited to) such topics as how you plan to manage your time to fit in your studies; how you will build your skills, as needed; how...
-
1. An introduction of you as a leader (whether or not you see yourself as a leader, whether or not you like being a leader, what kinds of leadership roles you have had, etc.). 2. Summarize your...
-
Briefly, describe the firm in terms of the following items. a. Size in terms of market capitalization, annual revenue, number of employees, location(s). b. Discuss the financial position of the firm....
-
HealthyLife (HL) is a publicly-traded company in the Food Manufacturing Industry. HealthyLife has been around since the 1970s, and is mainly focused on the production and wholesale of "organic and...
-
Suppose a maternal effect gene exists as a normal dominant allele and an abnormal recessive allele. A mother who is phenotypically abnormal produces all normal offspring. Explain the genotype of the...
-
Review Exhibit 11.4. Analyze each product on the graph according to the characteristics that influence the rate of adoption. For example, what can you conclude from the data about the relative...
-
Alkanes and aromatics are fairly stable compounds. To make them react, a special catalyst must be present. What catalyst must be present when reacting CI2 with an alkane or with benzene? Adding CI2...
-
The following are some other organic reactions covered in Section 21.4. Give an example to illustrate each type of reaction. a. Adding H2O to an alkene (in the presence of H+) yields an alcohol. b....
-
In the presence of light, chlorine can substitute for one (or more) of the hydrogens in an alkane. For the following reactions, draw the possible monochlorination products. hr 2,2-dimethylpropane Cl2...
-
When credit terms for a sale are 2/15, n/40, the customer saves by paying early. What percent (rounded) would this savings amount to on an annual basis
-
An industrial robot that is depreciated by the MACRS method has B = $60,000 and a 5-year depreciable life. If the depreciation charge in year 3 is $8,640, the salvage value that was used in the...
-
What determines a firm's beta? Should firm management make changes to its beta? Be sure to consider the implications for the firm's investors using CAPM.

Study smarter with the SolutionInn App


