Calculate the standard enthalpy of formation of dinitrogen pentoxide from the following data: and from the standard
Question:
Calculate the standard enthalpy of formation of dinitrogen pentoxide from the following data: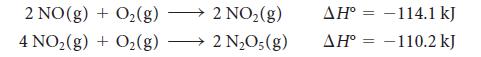
and from the standard enthalpy of formation of nitric oxide, NO.
Transcribed Image Text:
2 NO(g) + O2(g) + O2(g) 4 NO2(g) 2 NO2(g) 2 NO;(g) ΔΗ° = -114.1 kJ ΔΗ° = -110.2 kJ
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)

Answered By

Felix Onchweri
I have enough knowledge to handle different assignments and projects in the computing world. Besides, I can handle essays in different fields such as business and history. I can also handle both short and long research issues as per the requirements of the client. I believe in early delivery of orders so that the client has enough time to go through the work before submitting it. Am indeed the best option that any client that can think about.
4.50+
5+ Reviews
19+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Calculate the standard enthalpy of formation of NOCI (g) from the enthalpy of formation of NO given in Table 2.5, together with the following information: 2 NOCl (g) 2 NO (g) + Clz (g) 1 Ho = + Uo=...
-
Calculate the standard enthalpy of formation (DHf) of ClO from the following bond enthalpies: Cl2: 242.7 kJ/mol; O2: 498.7 kJ/mol; ClO: 206 kJ/mol.
-
Calculate the standard enthalpy of formation of FeS 2 (s) at 600. °C from the following data at 298.15 K. Assume that the heat capacities are independent of temperature. You are also given that...
-
Prestopino Corporation produces motorcycle batteries. Prestopino turns out 1,500 batteries a day at a cost of $6 per battery for materials and labor. It takes the firm 22 days to convert raw...
-
Hindi Company has the following production data for April: units transferred out 40,000, and ending work in process 5,000 units that are 100% complete for materials and 40% complete for conversion...
-
Evaluate the integral. (x + 1)
-
5. The December 31, 2016, balance sheet of the Ben, Car, and Das partnership is summarized as follows: Cash $100,000 Car loan $100,000 Other assets, at cost 500,000 Ben capital 100,000 Car capital...
-
In the past, Arup Mukherjees tire dealership in Pensacola sold an average of 1,000 radials each year. In the past 2 years, 200 and 250, respectively, were sold in fall, 350 and 300 in winter, 150 and...
-
eBook Print 1 incumete Net Present Value Analysis Cooper Company must evaluate two capital expenditure proposal Coopers hurdle rate is 10 Data for the two proposals follow ProposalX Proposal 120.000...
-
An important reaction that takes place in the atmosphere is NO 2 (g) NO(g) + O(g), which is brought about by sunlight. How much energy must be supplied by the Sun to cause it? Calculate the standard...
-
Use standard enthalpies of formation from Appendix 2A to calculate the standard reaction enthalpy for each of the following reactions: (a) The removal of hydrogen sulfide from natural gas: (b) The...
-
Write two conversion factors between nanoseconds (ns) and milliseconds (ms).
-
The four classic leadership styles There are four leadership styles which are prominent in today's businesses and companies. They are Laissez-faire, Autocratic, Democratic, and Charismatic...
-
How can conflict be viewed positively? Cite a specific example of when this might be the case. Under what circumstances might "avoiding" conflict be "managing" conflict? In other words, when might...
-
Visit the website and answer the questions below. https://www.forbes.com/advisor/business/software/best-crm-small-business/ Based on the CRM software discussed in the article, which 3 software...
-
Planning consists of translating and organizations mission and vision into objectives. The organization's purpose is expressed as a mission statement, and what it becomes is expressed as a vision...
-
Question 1- Visit the Boots and Hearts Festival website: www.bootsandhearts.com. Using the information you find on the site, make an analysis of the festival's Strengths, Weaknesses, Opportunities...
-
Suppose that a gene affects the anterior morphology in house flies and is inherited as a maternal effect gene. The gene exists in a normal allele, H, and a recessive allele, h, which causes a small...
-
Problem 3.5 (4 points). We will prove, in steps, that rank (L) = rank(LT) for any LE Rnxm (a) Prove that rank (L) = rank (LTL). (Hint: use Problem 3.4.) (b) Use part (a) to deduce that that rank(L) =...
-
Which of the following statements is (are) false? Explain why the starementfs) is (are) false. a. Is a structural isomer of pentonic acid. b. Is a structual isomer of 2-methyl-3-pentanone. c....
-
The following organic compounds cannot exist. Why? a. 2-chloro-2-butyne b. 2-methyl-2-propanone c. 1,1-dimethylbenzene d. 2-pentanal e. 3-hexanoic acid f. 5,5-dibromo-l-cyclobutanol
-
Mycomycin is a naturally occurring antibiotic produced by the fungus Nocardia acidophilus. The molecular formula of the substance is Q3H10O2, and its systematic name is...
-
Domino is 4 0 years old and is married out of community of property with the exclusion of the accrual system to Dolly ( 3 5 ) . They have one child, Domonique, who is 1 1 years old. Domino resigned...
-
YOU ARE CREATING AN INVESTMENT POLICY STATEMENT FOR JANE DOE General: 60 years old, 3 grown children that are living on their own and supporting themselves. She is in a very low tax rate so we don't...
-
firm purchased a new piece of equipment with an estimated useful life of eight years. The cost of the equipment was $65,000. The salvage value was estimated to be $10,000 at the end of year 8. Using...

Study smarter with the SolutionInn App


