Use data in Table 4H.1 or Appendix 2A to calculate the standard entropy change for each of
Question:
Use data in Table 4H.1 or Appendix 2A to calculate the standard entropy change for each of the following reactions at 25°C. For each reaction, interpret the sign and magnitude of the reaction entropy.
Table 4H.1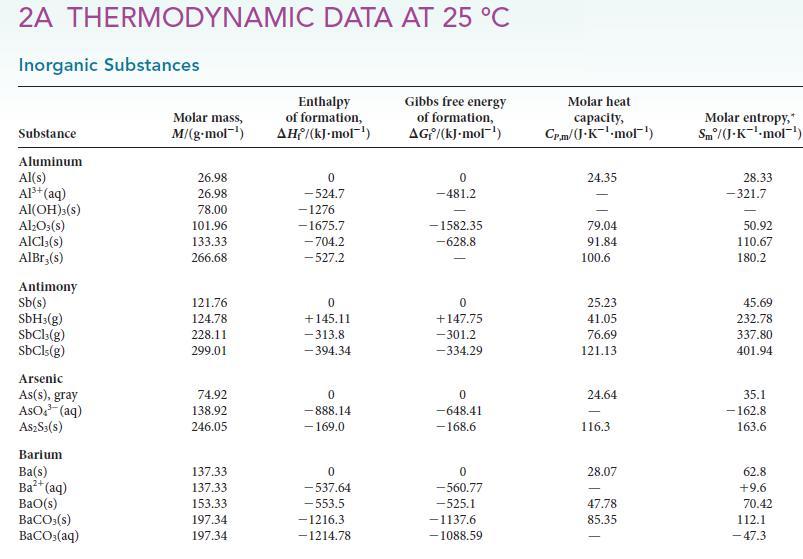
(a) The preparation of manganese metal in the thermite reaction: 4 Al(s) + 3 MnO2(s) → 3 Mn(s) + 2 Al2O3(s).
(b) A reaction used to power rockets: 7 H2O2(l) + N2H4(l) → 2 HNO3(aq) + 8 H2O(l).
(c) The purification of silicon: SiO2(s) + 2 C(s) → Si(s) + 2 CO(g).
(d) The preparation of nitric oxide:![]()
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:





