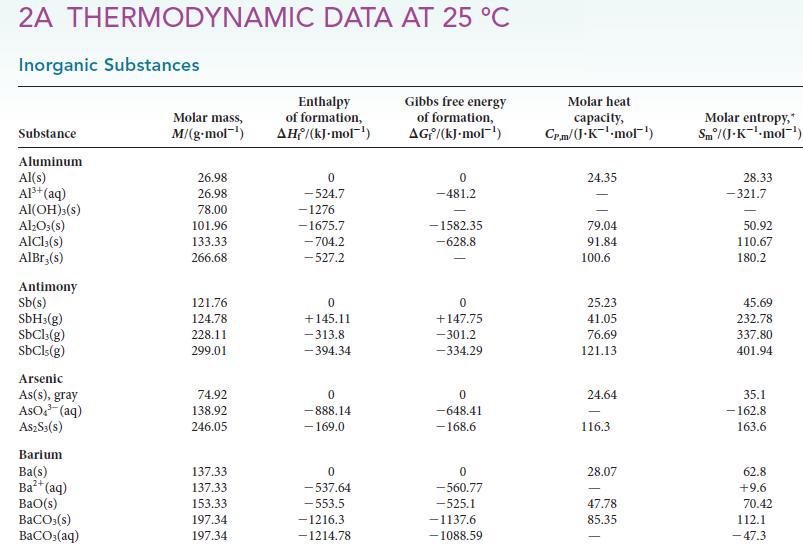
Use the standard Gibbs free energies of formation in Appendix 2A to calculate G for each of
Question:
Use the standard Gibbs free energies of formation in Appendix 2A to calculate ΔG° for each of the following reactions at 25 °C. Comment on the spontaneity of each reaction under standard conditions at 25 °C.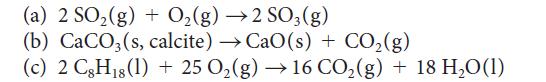
Transcribed Image Text:
(a) 2 SO₂ (g) + O₂(g) →2 SO3(g) (b) CaCO3(s, calcite) →CaO(s) + CO₂(g) (c) 2 CgHıs(l) + 25 O,(g) → 16 CO,(g) + 18 H,O(1) 18
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
a AG 14174 kJ mol ...View the full answer

Answered By

John Kago
Am a processional practicing accountant with 5 years experience in practice, I also happens to have hands on experience in economic analysis and statistical research for 3 years. am well conversant with Accounting packages, sage, pastel, quick books, hansa world, etc, I have real work experience with Strata, and SPSS
4.70+
31+ Reviews
77+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Use the standard Gibbs free energies of formation in Appendix 2A to calculate G for each of the following reactions at 25 C. Comment on the spontaneity of each reaction under standard conditions at...
-
Ammonia survives indefinitely in air. An agronomist studying how long ammonia survives in soil might need to know whether it survives because its oxidation is not spontaneous under ordinary...
-
Use data in Table 4H.1 or Appendix 2A to calculate the standard reaction entropy for each of the following reactions at 25C. For each reaction, interpret the sign and magnitude of the reaction...
-
Consider a scenario, in which there were four students (Arthur, Kevin, Morris, and Orlando) driving their cars back home from Houston. However, only Orlando was involved in a car accident. We want to...
-
Consider the electrostatic Green functions of Section 1.10 for Dirichlet and Neumann boundary conditions on the surface S bounding the volume V. Apply Green's theorem (1.35) with integration variable...
-
Use implicit differentiation to find z/x and z/y. x 2 + 2y 2 + 3z 2 = 1
-
Illustrating the relative importance of each assumption in developing marketability discounts for nonmarketable minority interests of business enterprises.
-
The statements of earnings for Pruitt Company summarized for a four- year period show the following (amounts in thousands of dollars): An audit revealed that in determining these amounts, the ending...
-
LU correct Find the expected value E(X) of the following data. Round your answer to one decimal place, I 0 1 1 2 3 P(X = x) 0.2 0.1 0.2 0.2 0.3 Answer How to enter your
-
(a) Consider the hydrogenation of benzene to cyclohexane, which takes place by the step-by-step addition of two H atoms per step: Draw Lewis structures for the products of the hydrogenation of...
-
Initially an ideal gas at 412 K occupies 12.62 L at 0.6789 atm. The gas is allowed to expand to 19.44 L by two pathways: (a) Isothermal, reversible expansion and (b) Isothermal, irreversible free...
-
Tell whether the two angles shown are complementary, supplementary, or neither. 10 9 11 12 1 8 7 5 6 2 3 4 10 9 11 12 8 7 6 5 N 3 4
-
Sunland Corp. exchanged Building 24, which has an appraised value of $1,815,000, a cost of $2,842,000, and accumulated depreciation of $1,272,000, for Building M which belongs to Oriole Ltd. Building...
-
Conlon Chemicals manufactures paint thinner. Information on the work in process follows: -Beginning Inventory, 43,000 partially complete gallons -Transferred out, 211300 gallons -Ending inventory...
-
Mr . Nikola Tesla launched Tesla Supermart on December 1 , 2 0 x 1 with a cash investment of 1 5 0 , 0 0 0 . The following are additional transactions for the month: 2 Equipment valued at 2 0 , 0...
-
The Robots: Stealing Our Jobs or Solving Labour Shortages? As the coronavirus pandemic enveloped the world, businesses increasingly turned to automation in order to address rapidly changing...
-
Aquazona Pool Company is a custom pool builder. The company recently completed a pool for the Drayna family ( Job 1 3 2 4 ) as summarized on the incomplete job cost sheet below. Assume the company...
-
Each molecule of hemoglobin, the oxygen carrier in blood, contains four Fe atoms. Explain how you would use the radioactive 2659Fe (t1/2 = 46 days) to show that the iron in a certain food is...
-
A supermarket chain is interested in exploring the relationship between the sales of its store-brand canned vegetables (y), the amount spent on promotion of the vegetables in local newspapers (x1)...
-
Breeder reactors are used to convert the nonfissionable nuclide 92 235 U to a fissionable product. Neutron capture of the 92 235 U is followed by two successive beta decays. What is the final...
-
The sun radiates 3.9 10 -3 J of energy into space every second. What is the rate at which mass is lost from the sun?
-
Fresh rainwater or surface water contains enough tritium ( 3 1 H) to show 5.5 decay events per minute per 100. g water. Tritium has a half-life of 12.3 years. You are asked to check a vintage wine...
-
Indicate whether the following managerial policy increases the risk of a death spiral:Use of low operating leverage for productionGroup of answer choicesTrueFalse
-
It is typically inappropriate to include the costs of excess capacity in product prices; instead, it should be written off directly to an expense account.Group of answer choicesTrueFalse
-
Firms can avoid the death spiral by excluding excess capacity from their activity bases. Group of answer choicesTrueFalse

Study smarter with the SolutionInn App


