Consider the ammonia synthesis reaction where G =33.3 kJ per mole of N 2 consumed at 25C.
Question:
Consider the ammonia synthesis reaction
![]()
where ΔG =–33.3 kJ per mole of N2 consumed at 25°C. For each of the
following mixtures of reactants and products at 25°C, predict the direction in
which the system will shift to reach equilibrium.
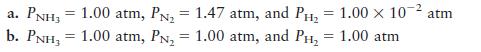
Transcribed Image Text:
N(g) + 3H(g) = 2NH3(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
a We can predict the direction of the shift to equilibrium by calculating the value of AG ...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Consider the reaction 2NO2(g) N2O4(g) For each of the following mixtures of reactants and products at 25oC, predict the direction in which the reaction will shift to reach equilibrium. Use...
-
For the synthesis of ammonia at 500C, the equilibrium constant is 6.0 10 2 L 2 /mol 2 . Predict the direction in which the system will shift to reach equilibrium in each of the following cases. -3 a....
-
What is the output of the program if the input value is 202 input_value = 0 input_value = int(input('Input value: ')) if input_value < 10: print('Live') elif input_value < 20: print('Long') elif...
-
By applying modern technology to agriculture, the United States has become the most productive food-producing nation in the world. The secret to solving the world food security problem lies in...
-
A particle moves in a medium under the influence of a retarding force equal to mk(v3 + a2v), where k and a are constants. Show that for any value of the initial speed the particle will never move a...
-
What are the three types of software attacks?
-
What were the outcomes and how did they compare with the objectives?
-
Which, if any, of the following scenarios would support an employees claim of discrimination on the basis of national origin? a. A Dominican chambermaid in a hotel is denied promotion to front-desk...
-
Qestion below pictures, can you help me to correct incremental cost approach in excel file? I know the answer should be NPV in favor of overhauling the old truck. 16% 1 Data Block 2 Bilboa...
-
The overall reaction for the corrosion (rusting) of iron by oxygen is Using the following data, calculate the equilibrium constant for this reaction at 25C. 4Fe(s) + 30(g) 2FeO3(s)
-
One method for synthesizing methanol (CH 3 OH) involves reacting gaseous carbon monoxide and hydrogen: Calculate G at 25C for this reaction, in which carbon monoxide gas at 5.0 atm and hydrogen gas...
-
Find $\mathrm{CVaR}_{h}(X)$ for the linear case of Exercise 3. Data from Exercises 3 Consider the position $X$ that has a uniform probability density between -40 and 60. Find...
-
Use a substitution of the form u= ax + b to evaluate the following indefinite integral. S3x 3x+4 dx
-
Task 3 In order to support other staff to complete future risk assessments, produce a short-written report that explains. how hazards that become risks can be controlled the importance of fully...
-
let arr = [x => x + 5, x => 8, x => x * 2]; let b = X; let a = arr.reduce((acc, f) => acc + f(b), 0); If we know a is 28, what's the value of X?
-
2. (10 pts.) Identify the point symmetry elements of the structures for which the given directions are equivalent. Enumerate the elements (i.e., the individual symmetry operations) that make up the...
-
Theory Newton's second law can be written in a more general form as where is the momentum of system of N objects and is the net external force on the system. This relationship says that the rate at...
-
A steam power plant operates on an ideal reheat- regenerative Rankine cycle with one reheater and two feedwater heaters, one open and one closed. Steam enters the high-pressure turbine at 15 MPa and...
-
SBS Company have received a contract to supply its product to a Health Care Service Hospital. The sales involve supplying 1,250 units every quarter, the sales price is RM 85 per unit. The Client...
-
The molar concentration of HCl in hydrochloric acid is reduced to 12% of its initial value by dilution. What is the difference in the pH values of the two solutions?
-
Salts are often used to create solutions with specific pH values. Suppose you need to prepare a salt solution with a pH of about 4.5 and have available sodium dihydrogen phosphate, NaH 2 PO 4 , and...
-
Calculate the standard reaction Gibbs free energy for the following cell reactions: 4+ (a) 2 Ce (aq) + 3I (aq) 2 Ce(aq) + 13 (aq), Ecell +1.08 V (b) 6 Fe+(aq) +2 Cr+(aq) + 7 HO(1) 6 Fe+ (aq) + CrO2...
-
Required information Skip to question [ The following information applies to the questions displayed below. ] Forten Company's current year income statement, comparative balance sheets, and...
-
Give a breakdown of all the intangible assets with the values shown in the statement of financial position of Unilever in 2022.
-
1-The yield to maturity will be greater than the coupon rate when a bond is selling at a premium. Select one: a. False b. True 2-Which one of the following would have the greatest present value,...

Study smarter with the SolutionInn App


