The overall reaction for the corrosion (rusting) of iron by oxygen is Using the following data, calculate
Question:
The overall reaction for the corrosion (rusting) of iron by oxygen is
![]()
Using the following data, calculate the equilibrium constant for this reaction at 25°C.
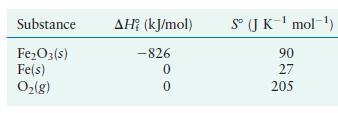
Transcribed Image Text:
4Fe(s) + 30(g) 2FeO3(s)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
We must first calculate ...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Find the value(s) of the variable. In Exercises 24-26, B and D are points of tangency. 14 7 C
-
You have fit a k-means algorithm with six clusters. The results are visualized below. Based on the plot, which statement is true? There is overlap between some clusters, which indicates there is no...
-
Synthesis Paper Write a three page synthesis paper that addresses the six trends and changes that Nagle cited. Use evidence from these four cases that explains the impact of these trends and changes...
-
Under Public Law 480, the United States sells surplus grains to developing countries, which pay in local currencies. Since the United States rarely spends all of these currencies, much of this grain...
-
A projectile is fired with initial speed v0 at an elevation angle of a up a hill of slope (a > ). (a) How far up the hill will the projectile land? (b) At what angle a will the range be a maximum?...
-
Describe the robotic revolution, and consider its possible implications for humans.
-
What can you learn from the evidence of this case?
-
Select the best answer for each of the following: 1. Johnson joined other creditors of Alpha Company in a composition agreement seeking to avoid the necessity of a bankruptcy proceeding against...
-
Blue Llama Mining Company's WACC is 10%, and the project has the same risk as the firm's average project. Calculate this project's modified internal rate of return (MIRR): 18.74% 20.53% -20.57%...
-
The value of K p is 3.7 10 6 at 900. K for the ammonia synthesis reaction. Assuming the value of H for this reaction is 92 kJ, calculate the value of K p at 550. K.
-
Consider the ammonia synthesis reaction where G =33.3 kJ per mole of N 2 consumed at 25C. For each of the following mixtures of reactants and products at 25C, predict the direction in which the...
-
How do reports created by OLAP differ from most conventional reports?
-
4. What is the time complexity of the following procedure for in/2 to n do j 2 end for while (j
-
If the concentration of a constituent in the influent to the equalization basin is constant over the 24 h period, will the load of the constituent from the basin be constant? If the concentration of...
-
A three-phase transmission line of a 60 Hz circuit has a length of 370 km (230 miles). the conductors are of the 795,000cm (54/7) type with horizontal spacing of 25 feet between them. The load on the...
-
Simulate rolling a dice using Math.random() . Your roll function should allow the caller to specify any number of sides, but default to 6 if no side count is given: roll() assumes a 6 sided dice,...
-
Drama Read the excerpt from a play. Then, answer the question(s). (1) (2) Belle: Having trouble deciding what will make you look like both a power to be reckoned with and a fetching young lady while...
-
Using EES (or other) software, investigate the effect of the boiler pressure on the performance of a simple ideal Rankine cycle. Steam enters the turbine at 500C and exits at 10 kPa. The boiler...
-
The area of square PQRS is 100 ft2, and A, B, C, and D are the midpoints of the sides. Find the area of square ABCD. B A
-
A student was given a standard Cu(s)|Cu 2+ (aq) half-cell and another half-cell containing an unknown metal M in 1.00 m M(NO 3 ) 2 (aq) and formed the cell M(s)|M + (aq)||Cu 2+ (aq)|Cu(s). The cell...
-
You are working in the research laboratory of a company developing new forms of batteries for installation in satellites. As a part of your investigation, you have decided to study various...
-
The images below represent the solutes in the solutions of three acids (water molecules are not shown, hydrogen atoms and hydronium ions are represented by small gray spheres, conjugate bases by...
-
A corporate bond that you own at the beginning of the year is worth $930. During the year, it pays $56 in interest payments and ends the year valued at $920. What was your dollar return and percent...
-
Anissa makes custom bird houses in her garage and she buys all her supplies from a local lumber yard. Last year she purchased $4500 worth of supplies and produced 2500 bird houses. She sold all 2500...
-
Critically comment on the following methods that have been used to account for goodwill i)Writting -off the cost of goodwill directly to reserves in the year of acquisition ii) Reporting goodwill at...

Study smarter with the SolutionInn App


