The images below represent the solutes in the solutions of three acids (water molecules are not shown,
Question:
The images below represent the solutes in the solutions of three acids (water molecules are not shown, hydrogen atoms and hydronium ions are represented by small gray spheres, conjugate bases by large colored spheres).
(a) Which acid is a strong acid?
(b) Which acid has the strongest conjugate base?
(c) Which acid has the highest pKa? Explain each of your answers.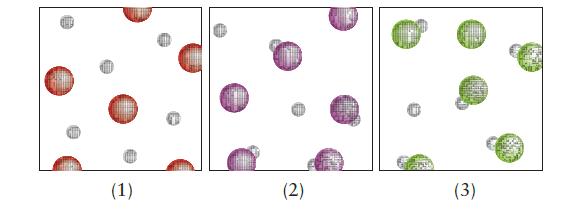
Transcribed Image Text:
HEL (1) (2) (3)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (3 reviews)
a Acid 1 is a str...View the full answer

Answered By

Ali Khawaja
my expertise are as follows: financial accounting : - journal entries - financial statements including balance sheet, profit & loss account, cash flow statement & statement of changes in equity -consolidated statement of financial position. -ratio analysis -depreciation methods -accounting concepts -understanding and application of all international financial reporting standards (ifrs) -international accounting standards (ias) -etc business analysis : -business strategy -strategic choices -business processes -e-business -e-marketing -project management -finance -hrm financial management : -project appraisal -capital budgeting -net present value (npv) -internal rate of return (irr) -net present value(npv) -payback period -strategic position -strategic choices -information technology -project management -finance -human resource management auditing: -internal audit -external audit -substantive procedures -analytic procedures -designing and assessment of internal controls -developing the flow charts & data flow diagrams -audit reports -engagement letter -materiality economics: -micro -macro -game theory -econometric -mathematical application in economics -empirical macroeconomics -international trade -international political economy -monetary theory and policy -public economics ,business law, and all regarding commerce
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
The images below represent the solutes in the solutions of three salts (water molecules are not shown, hydrogen atoms and hydronium ions are represented by small gray spheres, hydroxide ions by red...
-
Which of the following pictures best represents a solution made by adding 10 g of silver chloride, AgCl, to a liter of water? In these pictures, the gray spheres represent Ag+ ions and the green...
-
Which of the following pictures best represents an unsaturated solution of sodium chloride, NaCl? In these pictures, the dark gray spheres represent Na+ ions and the green spheres represent chloride...
-
The ABC Company has a cost of equity of 24.76 percent, a before-tax cost of debt of 5.37 percent, and a tax rate of 26 percent. What is the firm's weighted average cost of capital if the proportion...
-
Absorption and variable costing. (CMA) Osawa, Inc., planned and actually manufactured 200,000 units of its single product in 2009, its first year of operation. Variable manufacturing cost was $20 per...
-
How long does it take to prove KB | = using DPLL when is a literal already contained in KB? Explain.
-
Construct the graphics for evaluating the regression assumptions. Are they validated?
-
Two staff analysts for the city of Bridgmont, Pennsylvania, disagree regarding the effects of seniority and continuing education on promotions in the city government. One of them believes that...
-
Problem 4 Entries for payroll and payroll taxes Ch 1 0 The following information about payroll for the week ended December 3 1 was obtained from the records of Pelican Co . : Salaries: Deductions:...
-
Profitability remains a challenge for banks and thrifts with less than $ 2 billion of assets. The business problem facing a bank analyst relates to the factors that affect return on assets (ROA), an...
-
You are working in the research laboratory of a company developing new forms of batteries for installation in satellites. As a part of your investigation, you have decided to study various...
-
The molar concentration of HCl in hydrochloric acid is reduced to 12% of its initial value by dilution. What is the difference in the pH values of the two solutions?
-
What do you understand by contingency theory?
-
On July 1, 2021, P Company borrowed P160,000 to purchase 80 percent of the outstanding common stock of S Company. This loan, carrying a 10 percent annual rate, is payable in 8 annual installments...
-
Case Analysis Strategic leaders, being at the highest level of an organization, are responsible for charting its path to success. They visualize an ideal picture of their enterprise in a futuristic...
-
3 Refrigerant-134a enters a adiabatic compressor at 100 kPa and -24C with a flow rate of 1.300 m/min and leaves at 800 kPa and 60C. Determine the mass flow rate of R-134a and the power input to the...
-
The following trial balance of Bramble Traveler Corporation does not balance. Bramble Traveler Corporation Trial Balance April 30, 2025 Debit Credit Cash $6,221 Accounts Receivable 5,350 Supplies...
-
From this analysis, we can see than the actual number of unit produced was actually less than the forecasted, yet the actual revenue gain from were greater than the forecasted one. This situation...
-
Consider the data presented in Exercise 2.2.4. Construct a frequency distribution and display it as a table and as a histogram. Exercise 2.2.4 A dendritic tree is a branched structure that emanates...
-
What services are provided by the provincial and territorial governments?
-
The Heisenberg uncertainty principle says that the momentum and position of a particle cannot be known simultaneously and exactly. Can that information be obtained by measuring the momentum and...
-
Why isnt the motion of a human being described by the Schrdinger equation rather than Newtons second law if every atom in our body is described by quantum mechanics?
-
Explain the following statement: If h = 0, it would be possible to measure the position and momentum of a particle exactly and simultaneously.
-
*please calculate irr in excel
-
Which of the following would not be a period cost? Research and development Direct materials Office supplies Advertising costs
-
\ table [ [ Activity Cost Pool,Activity Measure,Total Cost,Total Activity ] , [ Machining , Machine - hours,$ 3 3 0 , 0 0 0 , 1 5 , 0 0 0 MHs ] , [ Machine setups,Number of setups,$ 3 0 0 , 0 0 0 , 5...

Study smarter with the SolutionInn App


