Dental amalgam, a solid solution of silver and tin in mercury, was used for filling tooth cavities.
Question:
Dental amalgam, a solid solution of silver and tin in mercury, was used for filling tooth cavities. Two of the reduction halfreactions that the filling can undergo are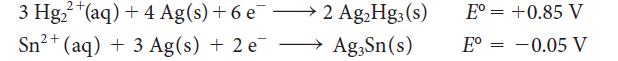
Suggest a reason why, if you accidentally bite on a piece of aluminum foil with a tooth containing a silver filling, you may feel pain.
Write a balanced chemical equation to support your suggestion.
Transcribed Image Text:
3 Hg+(aq) + 4 Ag(s) +6 e2 AgHg3 (s) 2+ Sn (aq) + 3 Ag(s) + 2 e Ag3Sn(s) E= +0.85 V -0.05 V E =
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
Consider Al aq 3 e Als E166 With this halfreaction ...View the full answer

Answered By

Sidharth Jain
My name is Sidharth. I completed engineering from National Institute of Technology Durgapur which is one of the top college in India. I am currently working as an Maths Faculty in one of the biggest IITJEE institute in India. Due to my passion in teaching and Maths, I came to this field. I've been teaching for almost 3 years.
Apart from it I also worked as an Expert Answerer on Chegg.com. I have many clients from USA to whom I teach online and help them in their assignments. I worked on many online classes on mymathlab and webassign. I guarantee for grade 'A'.
4.90+
3+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Herman is taking the U.S. History Advanced Placement Exam. He has taken 15 minutes to answer one short-answer question. Why is this a potential problem for Herman? He has 45 minutes to do two...
-
Some metal oxides, such as Sc2O3, do not react with pure water, but they do react when the solution becomes either acidic or basic. Do you expect Sc2O3 to react when the solution becomes acidic or...
-
The first stage in corrosion of iron upon exposure to air is oxidation to Fe2+. (a) Write a balanced chemical equation to show the reaction of iron with oxygen and protons from acid rain. (b) Would...
-
Energy of the emitted photon when an L-electron drops into the k-state in copper (z = 29) is -1 use R=109737 cm, cm =1.23910eV] 7994.6 eV 1094.6 eV 1293.6 eV 1097.3 eV
-
Analysis of return on invested assets, comparison of two divisions, DuPont method. Learning World, Inc. has two divisions: Test Preparation and Language Arts. Results (in millions) for the past three...
-
What is the magnitude of the magnetic dipole moment of the solenoid?
-
Describe the process of planning scope management? LO.1
-
Horace Society is planning its annual Western Fair Raceway Gala. The Gala committee has assembled the following expected costs for the event: Dinner (per person) . . . . . . . . . . . . . . . . . . ....
-
Which of the following statements are correct regarding the inclusion of a Crummey power in an ILIT? it makes the ILIT a grantor trust it limits the available annual exclusion to the lesser of the...
-
You have sent confirmations to 40 customers of Berg Shovick Express, a long-time audit client experiencing some financial difficulty. The company sells specialized high-technology goods. You have...
-
Use the data in Appendix 2B and the fact that, for the half-reaction F 2 (g) + 2 H + (aq) + 2 e 2 HF(aq), E = 13.03 V, to calculate the value of K a for HF. 2B STANDARD POTENTIALS AT 25 C...
-
(a) If you were to construct a concentration cell in which one half-cell contains 1.0 m CrCl 3 (aq) and the other half-cell contains 0.0010 m CrCl 3 (aq), and both electrodes were chromium, at which...
-
During the next four quarters, Dorian Auto must meet (on time) the following demands for cars: 4000 in quarter 1: 2000 in quarter 2:5000 in quarter 3. 1000 In quarter 4. At the beginning of quarter...
-
MAT 152 Project 3: MLB Team Salaries The data set below is the total salary of each Major League Baseball (MLB) team salaries per team in 2016. Find the probabilities for normal distributions and...
-
deficit, surplusincreased, decreased$795, $1,975, $54,635, $35 6. Cash-flow statement Sam and Joan Wallingford have been married for two years. They have been trying to save but feel that there is...
-
Trudy bought the vacant lot adjacent to her house and planted a large garden there. The garden produces more vegetables than her family needs, and Trudy earns some extra cash by selling them at a...
-
Requirements Medical researchers once conducted experiments to determine whether Lisinopril is a drug that is effective in lowering systolic blood pressure of patients. Patients in a treatment group...
-
1. Balroop while looking for Gurjap walks 315m [N] toward the forest, then 133 m [28 S of E] through it, and finally finds him deep inside the forest after walking another 55 m [ 31 S of W]....
-
In a study of preening behavior in the fruitfly Drosophila melanogaster, a single experimental fly was observed for 3 minutes while in a chamber with 10 other flies of the same sex. The observer...
-
A local politician is concerned that a program for the homeless in her city is discriminating against blacks and other minorities. The following data were taken from a random sample of black and...
-
Consider the reversible Carnot cycle shown in Figure 5.2 with 1.25 mol of an ideal gas with C V = 5/2R as the working substance. The initial isothermal expansion occurs at the hot reservoir...
-
The average heat evolved by the oxidation of foodstuffs in an average adult per hour per kilogram of body weight is 7.20 kJ kg 1 hr 1 . Assume the weight of an average adult is 62.0 kg. Suppose the...
-
Calculate S, S total , and S surroundings when the volume of 150. g of CO initially at 273 K and 1.00 bar increases by a factor of two in a. An adiabatic reversible expansion b. An expansion against...
-
Given that rJ = 6.3%, rRF = 4.1%, and rM = 9.4%, determine the beta coefficient for Stock J that is consistent with equilibrium.
-
Simon Companys year-end balance sheets follow. At December 31 2017 2016 2015 Assets Cash $ 33,019 $ 37,839 $ 38,623 Accounts receivable, net 93,822 65,556 54,152 Merchandise inventory 117,963 89,253...
-
PLEASE REFER TO THE 2018 ANNUAL REPORT OF STARBUKS FOR THE YEAR FISCAL YR 2018, ENDING SEPTEMBER 30, 2018. Refer to the management discussion & analysis section and write a one page summary...

Study smarter with the SolutionInn App


