Use the data in Appendix 2B and the fact that, for the half-reaction F 2 (g) +
Question:
Use the data in Appendix 2B and the fact that, for the half-reaction F2(g) + 2 H+(aq) + 2 e– → 2 HF(aq), E° = 13.03 V, to calculate the value of Ka for HF.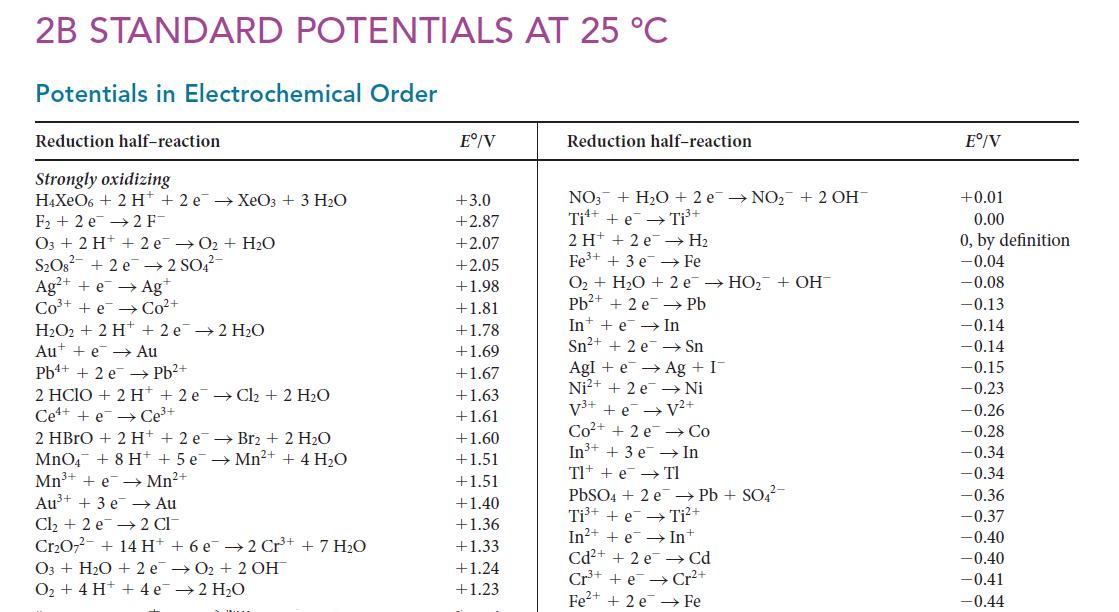
Transcribed Image Text:
2B STANDARD POTENTIALS AT 25 °C Potentials in Electrochemical Order Reduction half-reaction Strongly oxidizing H4XeO6+ 2 H+2 e → XeO3 + 3 H₂O F₂2 e 2 F- O3 + 2 H+ 2 e→O₂ + H₂O S₂O8² +2e →2 SO4²- Ag²+ +e → Agt Co³+ + e Co²+ H₂O2 + 2 H Au + e→→ Pb+ + 2 e + 2e →2 H₂O Au Pb²+ 2 HClO + 2 H+2 e → Cl₂ + 2 H₂O Cee Ce³+ →Mn²+ + 4H₂O 2 HBrO + 2 H+2 e Br2 + 2 H₂O MnO4 + 8 H+ + 5 e Mn³+ + e→ Mn²+ Au³+ + 3 e → Au Cl₂ + 2 e 2 CI Cr₂O7²- + 14 H+ + 6 e 2 Cr³+ + 7 H₂O O3+ H₂O + 2e →O₂ + 2 OH → 2H₂O O₂ + 4H+ + 4e Eº/V +3.0 +2.87 +2.07 +2.05 +1.98 +1.81 +1.78 +1.69 +1.67 +1.63 +1.61 +1.60 +1.51 +1.51 +1.40 +1.36 +1.33 +1.24 +1.23 Reduction half-reaction NO3 + H₂O + 2e →NO₂+ 2 OH Ti + e Ti³+ 2H+ +2 e Fe³+ + 3 e → H₂ Fe O₂ + H₂O + 2e → HO₂ + OH Pb²+ + 2 e pb In e In Sn²+ + 2 e AgI+ e → Sn Ag + I → Ni Ni²+ + 2e V³+ + e → V²+ Co²+ + 2e In³+ + 3 e Tl +e →→ Tl → Co In PbSO4 + 2 e Pb + SO4²- Ti³+ + e Ti²+ In²++eIn+ Cd²+ + 2 e Cd Cr³+ + eCr²+ Fe²+ + 2 e Fe Eº/V +0.01 0.00 0, by definition -0.04 -0.08 -0.13 -0.14 -0.14 -0.15 -0.23 -0.26 -0.28 -0.34 -0.34 -0.36 -0.37 -0.40 -0.40 -0.41 -0.44
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
2...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Use the data in Appendix 2B to calculate E(Ti 3+ /Ti). 2B STANDARD POTENTIALS AT 25 C Potentials in Electrochemical Order Reduction half-reaction Strongly oxidizing H4XeO6 + 2 H+2 e XeO3 + 3 HO F +2e...
-
Use the data in Appendix 2B to calculate E(U 4+ /U). 2B STANDARD POTENTIALS AT 25 C Potentials in Electrochemical Order Reduction half-reaction Strongly oxidizing H4XeO6 + 2 H+ 2 e XeO3 + 3 HO F+2 e...
-
Use only the data in Appendix 2B to calculate the acidity constant of HClO in water. 2B STANDARD POTENTIALS AT 25 C Potentials in Electrochemical Order Reduction half-reaction Strongly oxidizing...
-
Compute the stone's average velocity over the time interval [0.5, 2.5] and indicate the corresponding secant line on a sketch of the graph of h(t). A stone is tossed vertically into the air from...
-
ROI and RI. (D. Kleespie, adapted) The Outdoor Sports Company produces a wide variety of outdoor sports equipment. Its newest division, Golf Technology, manufactures and sells a single product:...
-
In Figure four long straight wires are perpendicular to the page, and their cross sections form a square of edge length a = 13.5 cm. Each wire carries 7.50 A, and the currents are out of the page in...
-
Understand the importance of good project scope management? LO.1
-
What type of research studies lend themselves to the use of e-mail for survey research? What are the advantages and disadvantages of using e-mail?
-
Rank the following assets from MOST Liquid (1) to LEAST Liquid (8): Gold _____ Common Stock _____ Houses _____ Houses _____ Checking Account _____ Automobiles _____ Currency _____ Camels _____...
-
Describe the three phases of the evolution of forecasting/prediction.
-
Suppose that 25.0 mL of a solution of Ag + ions of unknown concentration is titrated with 0.015 m KI(aq) at 25C. A silver electrode is immersed in this solution, and its potential is measured...
-
Dental amalgam, a solid solution of silver and tin in mercury, was used for filling tooth cavities. Two of the reduction halfreactions that the filling can undergo are Suggest a reason why, if you...
-
A process that emphasizes cross-functional integration and concurrent development of a product and its associated processes is known as _____. L0-1
-
Exhibit 12: Average Credit Quality Ratios [1] Based on this information you can compare O&Rs financial ratios to the average debt rating ratios above to assess what O&Rs credit rating would be if it...
-
Which of the following statements about QuickBooks Bill Pay are correct? Select all that apply. You can configure QuickBooks Bill Pay to pay bills automatically when they're added to QuickBooks...
-
Ash purchases 500 shares of XYZ for $10/share. Ten months later, when the shares are trading at $15/share, they donate them to Caring Trust, a qualified charity. Three months after the donation is...
-
Bob gets a X = 60 on his psychology exam and a X = 56 on his Biology exam. Psych exam scores had a =50 and =10 while Bio exam scores had a =48 and =4. Both professors grade on a curve. 1 - For which...
-
1. Lucky Company's direct labor information for the month of February is as follows: Actual direct labor hours worked (AQ) 61,500 Standard direct labor hours allowed (SQ) 63,000 Total payroll for...
-
Substances to be tested for cancer-causing potential are often painted on the skin of mice. The question arose Whether mice might get an additional dose of the substance by licking or biting their...
-
Use of the contraceptive Depo Provera appears to triple women's risk of infection with chlamydia and gonorrhea , a study reports today. An estimated 20 million to 30 million women worldwide use Depo...
-
The maximum theoretical efficiency of an internal combustion engine is achieved in a reversible Carnot cycle. Assume that the engine is operating in the Otto cycle and that C V ,m = 5/2 R for the...
-
The following two compounds each exhibit two heteroatoms (one nitrogen atom and one oxygen atom). In compound A, the lone pair on the nitrogen atom is more likely to function as a base. However, in...
-
2.25 moles of an ideal gas with C V ,m = 5/2 R is transformed from an initial state T 680. K and P = 1.15 bar to a final state T = 298.K and P = 4.75 bar. Calculate U, H, and S for this process.
-
Required : a- outline the statement of comperhensive income for the year ended 30 november 2021 b- outline the statment of financial position as at 30 November The Trial Balance of Alim Enterprise at...
-
International business and environment The MIR requires teams to gather current, or the most recently available, data on the markets people, economy, government, and technological status from online...
-
Consider the following stream of cash flows. The interest rate is 10%. 0 1 2 3 4 5 6 7 100 100 100 200 0 300 300 300 a) What is the value at time 0 of the cash flow stream? b) What is the value of...

Study smarter with the SolutionInn App


