Describe completely the galvanic cell based on the following half-reactions under standard conditions: Ag + e Fe+
Question:
Describe completely the galvanic cell based on the following half-reactions under standard conditions:
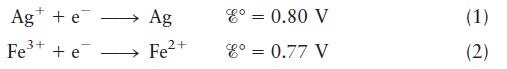
Transcribed Image Text:
Ag + e Fe+ + e Ag Fe2+ 8 = 0.80 V 8 = 0.77 V (1) (2)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
Since a positive cell value is required reaction 2 must run in 8 cathode 080 V 8 anode 077 ...View the full answer

Answered By

Vincent Omondi
I am an extremely self-motivated person who firmly believes in his abilities. With high sensitivity to task and operating parameters, deadlines and keen on instructions, I deliver the best quality work for my clients. I handle tasks ranging from assignments to projects.
4.90+
109+ Reviews
314+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Consider the standard galvanic cell based on the following half reactions Cu2+ + 2e- Cu Ag+ + e- Ag The electrodes in this cell are Ag(s) and Cu(s). Does the cell potential increase, decrease, or...
-
(A) Consider a galvanic cell based on the following half-reactions. Assuming the cell operates under standard conditions at 25 C, what is the spontaneous cell reaction? Under what nonstandard...
-
Consider the galvanic cell based on the following halfreactions: b. Calculate ÎGo and K for the cell reaction at 25oC. c. Calculate cell at 25oC when [Zn2+] = 0.10 M and [Fe2+] = 1.0 Ã...
-
Write a StockAccount client that builds an array of StockAccount objects, computes the total value of each account, and prints a report for the accounts with the largest and smallest values. Assume...
-
A particle of mass m has speed v = a/x, where x is its displacement. Find the force F(x) responsible.
-
The following table shows the annual returns on a portfolio of small stocks during the 20-year period from 1976 to 1995. What is the average return and standard deviation of this portfolio? How do...
-
1 3 Summarise at least one aspect of EU policy (or of an international trade agreement) which is of interest to you for your career
-
In 2010, its first year of operations, Kimble Corp. has a $900,000 net operating loss when the tax rate is 30%. In 2011, Kimble has $360,000 taxable income and the tax rate remains 30%. Instructions...
-
The following selected transactions relate to investment activities of Ornamental Insulation Corporation during 2021. The company buys debt securities, not intending to profit from short-term...
-
Using the data in Table 11.1, calculate G for the reaction Is this reaction spontaneous? Cu+ (aq) + Fe(s) Cu(s) + Fe+ (aq)
-
Consider the following galvanic cell: A 15.0-mol sample of \(\mathrm{NH}_{3}\) is added to the Ag compartment (assume 1.00 L of total solution after the addition). The silver ion reacts with ammonia...
-
Kamis Pink Purses buys and then resells a special type of pink purse. Here is some information concerning Kamis inventory activity during the month of August 2010: August 2860 units on hand at a...
-
Photon Technologies, Inc., a manufacturer of batteries for mobile phones, signed a contract with a large electronics manufacturer to produce three models of lithium-ion battery packs for a new line...
-
Mastery Problem: Capital Investment Analysis HomeGrown Company HomeGrown Company is a chain of grocery stores that are similar to indoor farmer's markets, providing fresh, local produce, meats, and...
-
McDonald's and CSR There more than 32,000 restaurants around the world (www.aboutmcdonalds.com/etc/medialib/csr/docs. that carry the McDonald's label and logo. As such, they...
-
Smartwatch Based on a survey by Consumer Technology Association, smartwatches are used in 18% of U.S. households. Find the probability that a randomly selected U.S. household has no smartwatches.
-
Suppose you wanted to purchase a commercial real estate property thats valued at $ 1 , 0 0 0 , 0 0 0 . You could secure financing from a traditional bank, which provides you with $ 7 5 0 , 0 0 0 ....
-
Consider a coal-fired steam power plant that produces 175 MW of electric power. The power plant operates on a simple ideal Rankine cycle with turbine inlet conditions of 7 MPa and 550C and a...
-
Extend Algorithms 3.4 and 3.5 to include as output the first and second derivatives of the spline at the nodes.
-
Citric acid, which is extracted from citrus fruits and pineapples, undergoes three successive deprotonations with pK a values of 3.14, 5.95, and 6.39. Estimate the pH of (a) A 0.15 m aqueous solution...
-
Is the criterion 6 3CO 2 ) 2 (aq) is found to be 9.11. However, the contribution to the pH from the autoprotolysis of water was ignored. Repeat the calculation of the pH of this solution, taking into...
-
Is the criterion 6 In Example 6D.4, the pH of 0.15 m NH 4 Cl(aq) is found to be 5.04. However, the contribution to the pH from the autoprotolysis of water was ignored. Repeat the calculation of the...
-
As a Financial Analyst in the Finance Department of Zeta Auto Corporation they are seeking to expand production. The CFO asks you to help decide whether the firm should set up a new plant to...
-
Chapter 4 When an Auditor finds misstatements in entities financial statements which may be the result of fraudulent act, what should be the role of an auditor under that situation? (2 Points)
-
Suppose the following input prices are provided for each year: Required: $

Study smarter with the SolutionInn App


