Question: Using the data in Table 11.1, calculate G for the reaction Is this reaction spontaneous? Cu+ (aq) + Fe(s) Cu(s) + Fe+ (aq)
Using the data in Table 11.1, calculate ΔG° for the reaction
![]()
Is this reaction spontaneous?
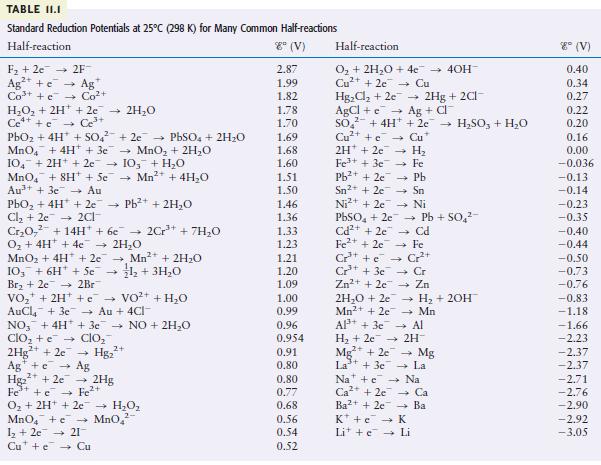
Cu+ (aq) + Fe(s) Cu(s) + Fe+ (aq)
Step by Step Solution
There are 3 Steps involved in it
The halfreactions are Cu 2e Cu Fe 2e Fe Cu Fe Cu Fe We can ... View full answer

Get step-by-step solutions from verified subject matter experts


