Question: Using the data from Table 11.1, predict whether 1 M HNO 3 will dissolve gold metal to form a 1 M Au 3+ solution. TABLE
Using the data from Table 11.1, predict whether 1 M HNO3 will dissolve gold metal to form a 1 M Au3+ solution.
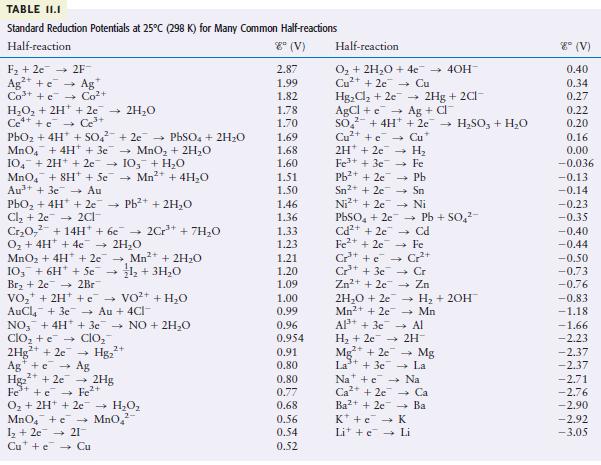
TABLE II.I Standard Reduction Potentials at 25C (298 K) for Many Common Half-reactions Half-reaction 8 (V) F + 2e 2F Ag+ + Ag+ Co+ + e Co+ HO + 2H+ + 2e 2HO C++ c Ce + PbO + 4H+ + SO4 MnO4 + 4H+ + 3e 104 + 2H+ + 2c 103 + HO MnO + 8H + 5e Mn+ + 4HO Au++ 3e Au +2e PbSO4 + 2HO MnO + 2HO PbO + 4H* + 2e Pb+ + 2HO Cl +2e 2Cl- Cr0 +14H+ + 6e 2Cr+ + 7HO O + 4H+ + 4e 2HO MnO + 4H+ + 2e Mn+ + 2HO 103 + 6H + Se + 3HO Br +2e 2Br VO + 2H+ VO+ + HO AuCl + 3e Au + 4Cl- + NO3 + 4H+3e7 NO + 2HO ClO + e clo 2Hg+ + 2e Hg+ Ag Age Hg+2e7 2Hg Fe + c Fe+ 3+ O + 2H+ + 2e HO. MnO4 + MnO 1 +2e 21 Cu + e Cu 2.87 1.99 1.82 1.78 1.70 1.69 1.68 1.60 1.51 1.50 1.46 1.36 1.33 1.23 1.21 1.20 1.09 1.00 0.99 0.96 0.954 0.91 0,80 0.80 0.77 0.68 0.56 0.54 0.52 Half-reaction O + 2HO + 4e Cu+ + 2e7 Cu HgCl +2e AgCl +eAg + Cl SO Cu+ + Cu* Ni+ + 2e PbSO4 + 2e Cd+ + 20 Fe+ + 2e + 4H+ + 2e -> HSO3 + HO 2H + 2e7 H Fe+ + 3e Fe Pb+ + 2e Pb Sn+ + 2e Sn Ni Cr+ + e Cr+ + 3e Zn+ + 2e 2HO + 2e Mn2+ + 2e 2Hg + 2Cl AP+ + 3e H +2e Mg+ + 2e La+ + 3e Cr+ Cr Zn - Pb + SO4- Cd Fe 40H H + 2OH Mn Al 2H Mg La Na + e Na Ca+2e Ca Ba+ + 2e Ba K + e K Li + c Li 8 (V) 0.40 0.34 0.27 0.22 0.20 0.16 0.00 -0.036 -0.13 -0.14 -0.23 -0.35 -0.40 -0.44 -0.50 -0.73 -0.76 -0.83 -1.18 -1.66 -2.23 -2.37 -2.37 -2.71 -2.76 -2.90 -2.92 -3.05
Step by Step Solution
3.37 Rating (150 Votes )
There are 3 Steps involved in it
The halfreaction for HNO3 acting as an oxidizing agent i... View full answer

Get step-by-step solutions from verified subject matter experts


