Determine the formal charge on each atom in the following ions. Identify the structure of lowest energy
Question:
Determine the formal charge on each atom in the following ions. Identify the structure of lowest energy in each case.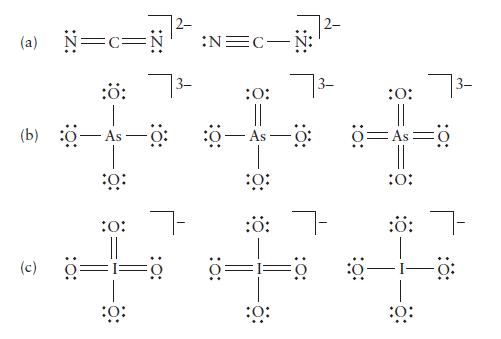
Transcribed Image Text:
(a) N=c= -Ö: (9) (c) :Ö: 0: :0: 7- :Ö: :N=C- :O: As :0: :ö: 7- ö= :0: :O: Ö=As :0: :Ö: :0:
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
To determine the formal charge on each atom in the given ions well use the formula Formal charge Number of valence electrons in free atom Number of nonbonding electrons Number of bonds Lets apply this ...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Determine the formal charge on each atom in the following molecules. Identify the structure of lower energy in each pair. (a) =-: || | :0: H (b) =c=S (c) H-C=N: :0a: T :0: :8-c=s: | H H-CIN
-
Two contributions to the resonance structure are shown below for each species. Determine the formal charge on each atom and then, if possible, identify the Lewis structure of lower energy for each...
-
Create Common Size Analysis (i.e., vertical financial analysis) for Facebook and Twitter This should be a providing an Income Statement Common Size Analysis (CSA) for Facebook and one key competitor...
-
Attlee Ltd holds 28% of the issued shares of Nehru Ltd. Attlee Ltd acquired these shares on 1 July 2019 and on this date all the identifiable assets and liabilities of Nehru Ltd were recorded at...
-
The management of Mid South Utilities Inc. is considering two capital investment projects. The estimated net cash flows from each project are as follows: The generating unit requires an investment of...
-
Find a vector equation and parametric equations for the line. The line through the point (5, 7, 1) and perpendicular to the plane 3x 2y + 2z = 8
-
Define extrapolation.
-
Hovington, CPA, knows that while audit objectives relating to inventories may be stated in terms of the assertions as presented in this chapter, they may also be subdivided and stated more...
-
Shamrock Farms provided the following expense information for April: Assembly-line workers' wages Plastic milk bottles Delivery expenses Caps for milk bottles Reconfiguring the assembly line...
-
Give the ground-state electron configuration and number of unpaired electrons expected for each of the following ions: (a) Ca 2+ ; (b) In + ; (c) Te 2- ; (d) Ag + .
-
Ionic compounds typically have higher boiling points and lower vapor pressures than covalent compounds. Predict which compound in the following pairs has the lower vapor pressure at room temperature:...
-
You want to install sod on one-half of your parallelogram-shaped backyard as shown. The patio covers the other half. Sod costs \(\$ 50\) a bag and covers \(25 \mathrm{ft}^{2}\). How much will it cost...
-
+ Given f(x) = x - 9 and g(x) = x+9, complete the following. (a) Find f(g(x)) and g(f(x)). (Simplify your answers completely.) f(g(x)) = g(f(x)) = (b) What does this tell us about the relationship...
-
Case Study - Rhonda Rhonda is a 28-year-old woman who has been referred to your agency by a local probation officer. Rhonda reported that she has "fired" three counselors in the past and most...
-
Calculating depreciationpartial periods LO2, 3 West Coast Tours runs boat tours along the west coast of British Columbia. On March 5, 2020, it purchased, with cash, a cruising boat for $936,000,...
-
Question 1. Write down the form of partial fractions needed to decompose the following: 482+2 (a) s32s24s 482+2 (c) s36s20 482 +2 - 4s8 (b) 8. 3 - 282 482+2 (d) s3 +2s2 - 2 Note: You are not being...
-
On December 31, 2022, Ace Hardware reported the following information on its balance sheet Accounts Receivable Allowance for Doubtful Accounts $900,000 $54,000 (credit) During 2023, the Company had...
-
A 0.450-g sample of steel contains manganese as an impurity. The sample is dissolved in acidic solution and the manganese is oxidized to the permanganate ion MnO4-. The MnO4-ion is reduced to Mn2+ by...
-
If the joint cost function for two products is C(x, y) = xy2 + 1 dollars (a) Find the marginal cost (function) with respect to x. (b) Find the marginal cost with respect to y.
-
When phosphorus reacts with excess chlorine gas, the compound phosphorus pentachloride (PCl 5 ) is formed. In the gaseous and liquid states this substance consists of PCl 5 molecules, but in the...
-
Describe the molecular structure of the water molecule.
-
Give possible Lewis structures for XeO 3 , an explosive compound of xenon. Determine the formal charges of each atom in the various Lewis structures.
-
thumbs up if correct A stock paying no dividends is priced at $154. Over the next 3-months you expect the stock torpeither be up 10% or down 10%. The risk-free rate is 1% per annum compounded...
-
Question 17 2 pts Activities between affiliated entities, such as a company and its management, must be disclosed in the financial statements of a corporation as O significant relationships O segment...
-
Marchetti Company, a U.S.-based importer of wines and spirits, placed an order with a French supplier for 1,000 cases of wine at a price of 200 euros per case. The total purchase price is 200,000...

Study smarter with the SolutionInn App


