Two contributions to the resonance structure are shown below for each species. Determine the formal charge on
Question:
Two contributions to the resonance structure are shown below for each species. Determine the formal charge on each atom and then, if possible, identify the Lewis structure of lower energy for each species.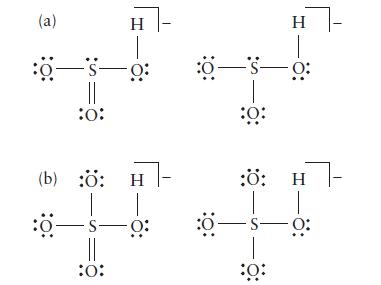
Transcribed Image Text:
(a) :0-S- 0: :0: H (b) :Ö: H :0-S- :0: H :0—5—0: :Ö: :: H 5—s—0: :Ö: - 0:
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
a b TO H o OSO 00 l...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Two Lewis structures are shown below for each species. Determine the formal charge on each atom and then, if appropriate, identify the Lewis structure of lower energy for each species. (a)...
-
Stratospheric ozone, O 3 , protects life on Earth from harmful ultraviolet radiation from the Sun. Suppose you are an atmospheric chemist; to understand the spectroscopic and structural properties of...
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
Head movement evaluations are important because disabled individuals may be able to operate communications aids using head motion. The paper Constancy of Head Turning Recorded in Healthy Young Humans...
-
Steamboat Co. expects to maintain the same inventories at the end of 2010 as at the beginning of the year. The total of all production costs for the year is therefore assumed to be equal to the cost...
-
What does it mean to say that marketing communications should be directed, ultimately, at affecting behavior rather than merely enhancing equity? Provide an example to support your answer.
-
Costs and expenses are the same. State whether the following statements are true or false:
-
Are marketers to blame for increasing obesity rates among children? Should the government ban the advertising of food products to children ages 17 and younger? Discuss the consequences of imposing...
-
Wyman Corporation is a service company that measures its output by the number of customers served. The company has provided the following fixed and variable cost estimates that it uses for budgeting...
-
Give the valence-shell electron configurations and bond orders for NO and NO + . Use that information to predict which species has stronger bonds.
-
Place the following molecules or ions in order of decreasing bond order: (a) NO bond in NO, NO 2 , NO 3 ; (b) CC bond in C 2 H 2 , C 2 H 4 , C 2 H 6 ; (c) CO bond in CH 3 OH, CH 2 O, CH 3 OCH 3 ....
-
Explain a financial ratio and then identify the following ratios, indicating what they are supposed to test. How would you evaluate each, given its specific numbers? After you complete that...
-
Lazlo s estimates uncollectible accounts to be 0 . 9 % of sales. Its year - end unadjusted trial balance shows Accounts Receivable of $ 1 1 2 , 5 0 0 and sales of $ 9 6 5 , 0 0 0 . If Lazlo s uses...
-
Identify one or two of the best and one or two of the worst work teams on which you served as a member. 1. Identify the top three to five factors that made the team the best or the worst in terms of...
-
ColorCoder is a HousePaint Shop which supplies currently two types of house paints, namely, alpha and beta house paints. The shop is planning to sell a primer (paint base) and the needed paint...
-
which department adds value to a product or service that is observable by a customer?
-
Using Figure 14.1, answer the following questions: a. What was the settle price for July 2022 coffee futures on this date? What is the total dollar value of this contract at the close of trading for...
-
In a certain electrolysis experiment involving Al3+ ions, 60.2 g of Al is recovered when a current of 0.352 A is used. How many minutes did the electrolysis last?
-
Apply Jacobis method to the given system. Take the zero vector as the initial approximation and work with four-significant-digit accuracy until two successive iterates agree within 0.001 in each...
-
Draw the conjugate acid for each of the following bases: (a) (b) (c) NaNH 2 (d) H 2 O (e) (f) (g) (i) NaOH
-
How many stereo-isomers do you expect for the following compound? Draw all of the stereo isomers. ,
-
Propose an efficient synthesis for each of the following transformations. You might find it useful to review Section 11.13 before doing these problems. In section 11.13 Radical halogenation provides...
-
Indicate whether the following managerial policy increases the risk of a death spiral:Use of low operating leverage for productionGroup of answer choicesTrueFalse
-
It is typically inappropriate to include the costs of excess capacity in product prices; instead, it should be written off directly to an expense account.Group of answer choicesTrueFalse
-
Firms can avoid the death spiral by excluding excess capacity from their activity bases. Group of answer choicesTrueFalse

Study smarter with the SolutionInn App


