Determine the temperature change when 4.00 g of (a) KCl; (b) MgBr 2 ; (c) KNO 3
Question:
Determine the temperature change when 4.00 g of
(a) KCl;
(b) MgBr2;
(c) KNO3;
(d) NaOH is dissolved in 100. g of water.
Assume that the specific heat capacity of the solution is 4.18 J · K–1 · g–1 and that the enthalpies of solution in Table 5D.3 are applicable.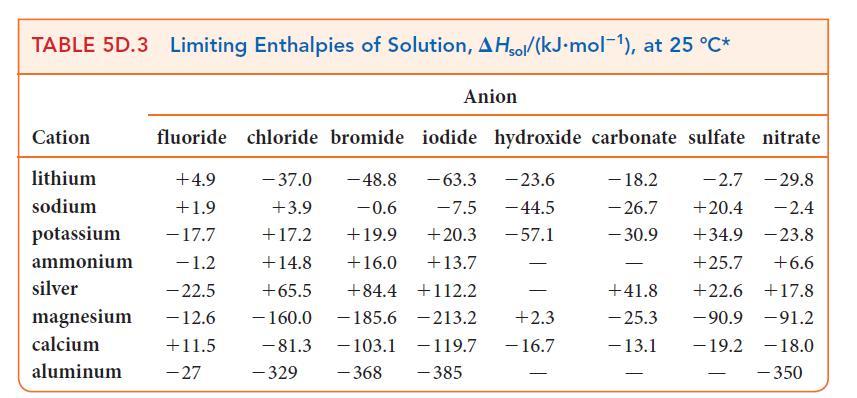
Transcribed Image Text:
TABLE 5D.3 Limiting Enthalpies of Solution, AHsol/(kJ.mol-1), at 25 °C* Anion Cation lithium sodium fluoride chloride bromide iodide hydroxide carbonate sulfate nitrate +4.9 - 37.0 -48.8 - 23.6 - 18.2 -2.7 - 29.8 -63.3 -7.5 - 44.5 +1.9 +3.9 -0.6 - 26.7 +20.4 -2.4 +34.9 -23.8 potassium - 17.7 +17.2 +19.9 +20.3 -57.1 - 30.9 ammonium -1.2 +14.8 +16.0 +13.7 +25.7 +6.6 silver -22.5 +65.5 +84.4 +112.2 +22.6 +17.8 magnesium - 12.6 - 160.0 -185.6 213.2 -90.9 -91.2 calcium +11.5 -19.2 -18.0 -81.3 -329 - 368 -103.1 - 119.7 -385 aluminum -27 - +2.3 - 16.7 - +41.8 - 25.3 - 13.1 - - 350
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
To determine the temperature change when a substance is dissolved in water we can use the equation T q m C where T is the change in temperature q is t...View the full answer

Answered By

Carly Cimino
As a tutor, my focus is to help communicate and break down difficult concepts in a way that allows students greater accessibility and comprehension to their course material. I love helping others develop a sense of personal confidence and curiosity, and I'm looking forward to the chance to interact and work with you professionally and better your academic grades.
4.30+
12+ Reviews
21+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Assume that 50 g of KCl is dissolved in 100 g of water at 100 C and the solution is allowed to cool to 20 C. (a) How much KCl remains in solution? (b) How much KCl crystallizes from the solution?
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
For the calculation of the solubility c of a gas in a solvent, it is often convenient to use the expression c=Kp, where K is the Henry's law constant. Breathing air at high pressures, such as in...
-
Air temperature usually decreases with increasing altitude. However, during the winter, thanks to a phenomenon called thermal inversion, the temperature of air warmed by the sun in mountains above a...
-
In Figure, the emf is 6 V and R = 0.5 ?. The rate of Joule heating in R is 8 W. (a) What is the current in the circuit? (b) What is the potential difference across R? (c) What is r? & W+|1 www R
-
Given the stresses in psi: xx = 1,500 yy = 2,000 zz = 3,500 xy = 600 yz = 300 zx = 500 Where x = east, y = north, z = up, compression is positive, find: the secondary principal stresses in the...
-
(Multiple-step and Single-step) Two accountants for the firm of Elwes and Wright are arguing about the merits of presenting an income statement in a multiple-step versus a single-step format. The...
-
The trial balance of Roman Company at the end of its fiscal year, August 31, 2014, includes these accounts: Inventory $17,200; Purchases $149,000; Sales Revenue $190,000; Freight-In $5,000; Sales...
-
Question 1 On January 1 , 2 0 2 1 , A Ltd . signed a 1 0 - year non - cancelable lease agreement to lease a storage building from S Ltd . The following information pertains to this lease agreement....
-
Example of Epic Proportions Twenty-five years ago Ronaldo, an accomplished cyclist, purchased a small custom carbon fiber wheel builder Zircles Inc. He applied their expertise in carbon fiber to...
-
Calculate the osmotic pressure at 20C of each of the following solutions, assuming complete dissociation of ionic compounds: (a) 4.5 * 10 3 m C 6 H 12 O 6 (aq); (b) 3.0 * 10 3 m CaCl 2 (aq); (c)...
-
Calculate the equilibrium constant at 25C and at 100C for each of the following reactions, using data available in Appendix 2A: (a) 2 CuO (s) 2 Cu(s) + O(g) (b) CH4(g) + H(g) CH6(g)
-
Consider a solar-energy-powered ideal Rankine cycle that uses water as the working fluid. Saturated vapor leaves the solar collector at 350 F, and the condenser pressure is 1 lbf/in.2. Determine the...
-
Case # 4 Joseph Joseph, a 19-year-old African American college freshman. Yesterday he spent the afternoon drinking beer and taking shots of vodka with his fraternity brothers. After 6 glasses of beer...
-
1. Kaldor facts [50 points] Kaldor (1961) documented a set of stylized facts on the growth process of industrialized countries. We discussed these facts in lecture 2. Explain if and how the...
-
County has the Investment Activities recorded in its general fund: Tesla Stock: Cost $100, Fair Value on Jan 1x1: $200; Fair Value on Dec 31x2: $300 DJT Stock: Cost: $100; Fair Value on Jan 1x1:...
-
Pets World is a retailer of a popular blend of organic dog food produced by Natural Pets Company. On average, Pets World sells 600 cans per week. The wholesale price that Natural Pets Company charges...
-
Out Supply-Chaining the King of Supply Chainers, How easy (or hard) would it be for rivals like Walmart or Carrefour to adopt Tesco's data management techniques? (Please provide reference...
-
In Figure P2.4.7, a single-reading mercury manometer is used to measure water pressure in the pipe. What is the pressure (in psi) if h1 = 6.9 in and h2 = 24.0 in.? h2 Figure P2.4.7
-
Continuation of Exercise 4-83. (a) What is the probability that the first major crack occurs between 12 and 15 miles of the start of inspection? (b) What is the probability that there are no major...
-
Identify the reagents that you would use to achieve each of the following transformations: a. b. Br Br
-
Discuss the following statement: Heating an object causes its temperature to increase.
-
The Joule coefficient is defined by (T /V) U = (1/C V )[P T(P/T) V ]. Calculate the Joule coefficient for an ideal gas and for a van der Waals gas.
-
During the month of September,the Cider Pressing Company is trying to determine how much cider they are going to sell in October and November. One gallon of cider typically sells for $7 per gallon....
-
This is very confusing please help with descriptions if possible. Complete this question by entering your answers in the tabs below. Prepare a master budget for the three-month period ending June 30...
-
Doug recibe un dplex como regalo de su to. La base del to para el dplex y el terreno es de $90,000. En el momento de la donacin, el terreno y el edificio tienen un FMV de $40 000 y $80 000,...

Study smarter with the SolutionInn App


