Calculate the equilibrium constant at 25C and at 100C for each of the following reactions, using data
Question:
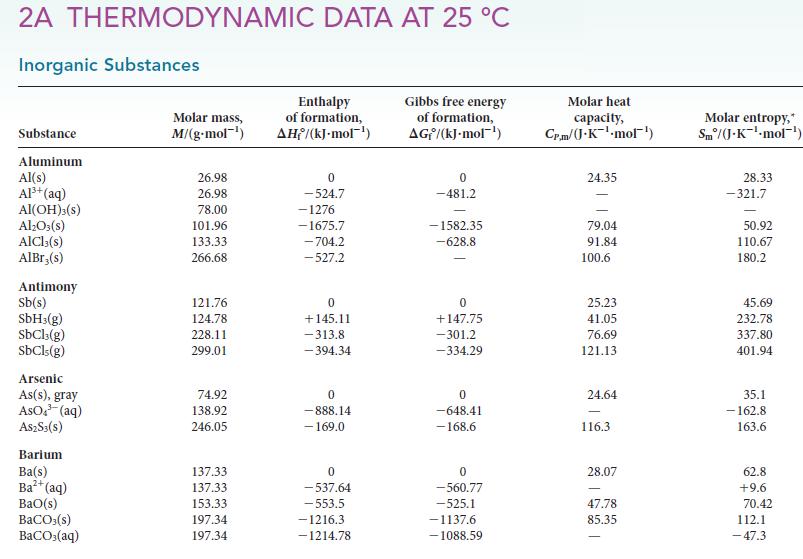
Calculate the equilibrium constant at 25°C and at 100°C for each of the following reactions, using data available in Appendix 2A: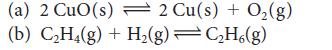
Transcribed Image Text:
(a) 2 CuO (s) 2 Cu(s) + O(g) (b) CH4(g) + H(g) CH6(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
Because we want the equilibrium constant at two temperatures we will need to calculate AH and ASo fo...View the full answer

Answered By

Caroline Kinuthia
Taking care of the smaller details in life has a larger impact in our general well being, and that is what i believe in. My name is Carol. Writing is my passion. To me, doing a task is one thing, and delivering results from the task is another thing. I am a perfectionist who always take things seriously and deliver to the best of my knowledge.
4.90+
1934+ Reviews
4277+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
The budgeted income statement for Barnaby's Hideaway is produced on your Excel spreadsheet. Assume that the following constitute the fixed and vari- able costs for the upcoming year Fixed Costs for...
-
Calculate the equilibrium constant Kc at 25oC from the free-energy change for the following reaction: See Appendix C for data. Zn(s) +2Ag (a)Zn2 (a) Ag(s)
-
Calculate the equilibrium constant at 25C and at 150C for each of the following reactions, using data available in Appendix 2A: (a) NH4Cl(s) (b) H(g) + DO(1) NH3(g) + HCl (g) D(g) + HO(1)
-
In Problems 5978, solve each equation in the complex number system. x 2 + 25 = 0
-
Nine 10-? resistors are connected as shown in Figure, and a potential difference of 20 V is applied between points a and b. (a) What is the equivalent resistance of this network? (b) Find the current...
-
Given the strains: xx = 2,000 yy = 3,000 zz = 4,500 xy = 200 yz = 300 zx = 225 Where x = east, y = north, z = up, compression is positive, the units are micro inches per inch, Youngs modulus E =...
-
(Income Statement Items) Presented below are certain account balances of Paczki Products Co. Instructions From the foregoing, compute the following: (a) total net revenue, (b) net income, (c)...
-
Discuss the risks depicted by the fixed asset system flowchart for Problem 10. Describe the internal control improvements to the system that are needed to reduce theserisks. Vendor User Department...
-
The International Accounting Standards Board: 31 2 W Multiple Choice S X mand Can overrule the FASB when their policies disagree. #3 Is governed by the U.S. Securities and Exchange Commission. Is the...
-
Let us consider the a small village. The power network of the village is shown in Figure 1. Load demands at Zone A, Zone B and Zone C, are 5MVA (0.95pf lagging), 7MVA (0.94pf lagging) and 7MVA...
-
Determine the temperature change when 4.00 g of (a) KCl; (b) MgBr 2 ; (c) KNO 3 ; (d) NaOH is dissolved in 100. g of water. Assume that the specific heat capacity of the solution is 4.18 J K 1 g 1...
-
When solid NH 4 HS and 0.400 mol NH 3 (g)were placed in a vessel of volume 2.0 L at 24C, the equilibrium NH 4 HS(s) NH 3 (g)+ H 2 S(g), for which Kc = 1.6 * 10 4 , was reached. What are the...
-
Meals on Wheels are considered two contracts to renovate its facilities. The first contractor wants $20,000 for the job and, with a maintenance fee of $1,000 per year, will guarantee the repairs for...
-
Compare the alternatives that Bergerac is considering for its decision. Include: Comparison of make versus buy option in the type of operation that Bergerac is looking to integrate. You do not need...
-
Let A, B, C and D be non-zero digits, such that CD is a two-digit positive integer. BCD is a three-digit positive integer generated by the digits B, C and D. ABCD is a four-digit positive integer...
-
1.) An aluminum tube is clamped with rigid plates using four bolts as shown. The nut on each bolt is tightened one turn from 'snug'. The thickness of the plate may be considered insignificant in this...
-
4.21 Case Study Competency IV.1RM Determine diagnosis and procedure codes and groupings according to official guidelines. Competency IV.1 Validate assignment of diagnostic and procedural codes and...
-
W.E.B Dubois taught the book called "The State" to his students at Atlanta University. Who wrote this book
-
An open manometer, shown in Figure P2.4.6, is installed to measure pressure in a pipe carrying an ii (sp. gr. = 1.60). If the monometer liquid is carbon tetrachloride (sp. gr. = l. 60), determine the...
-
Write a paper about the Working relationship in the organization- collaboration within and outside the organization
-
What is the physical origin of the pressure difference across a curved liquidgas interface?
-
Draw the mechanism for each of the following transformations: a. b. c. Br HBr
-
Using the result of Equation (3.8), (P/T) V = / , express as a function of and V m for an ideal gas, and as a function of b, , and V m for a van der Waals gas.
-
FINANCIAL STATEMENT ANALYSIS INSTRUCTIONS 1. PREPARE RATIO ANALYSIS REPORT ( word file) Format 1. Introduction 2. Importance of Financial Statements 3. Importance of Financial statement analysis and...
-
Let us assume that Europe is in recession, China's economy is slowing down, and the US economy is growing at 1-2%. Use these assumptions to invest in 4 ETFs (electronically traded funds). The 4 ETFs...
-
A section 83(b) election creates ordinary income at the time of the grant. Ture or False

Study smarter with the SolutionInn App


