Calculate the equilibrium constant at 25C and at 150C for each of the following reactions, using data
Question:
Calculate the equilibrium constant at 25°C and at 150°C for each of the following reactions, using data available in Appendix 2A:![]()
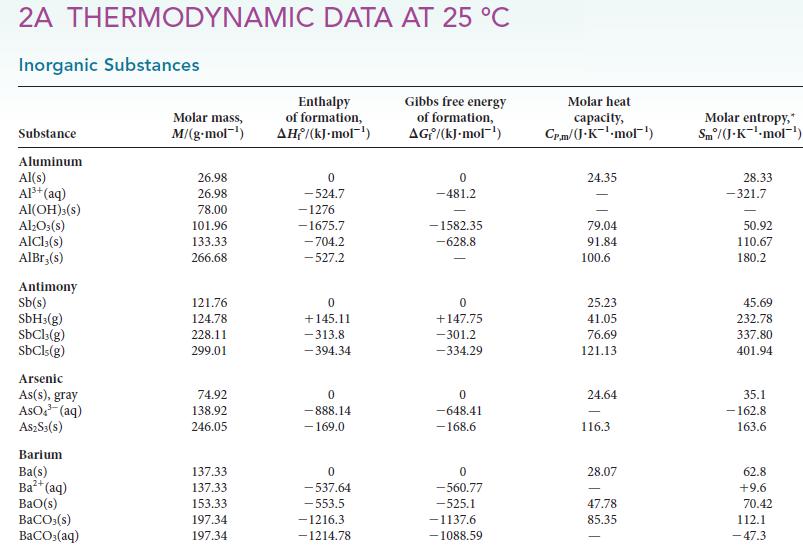
Transcribed Image Text:
(a) NH4Cl(s) (b) H₂(g) + D₂O(1) NH3(g) + HCl (g) D₂(g) + H₂O(1)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
a At 298 K K 1 X ...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
The budgeted income statement for Barnaby's Hideaway is produced on your Excel spreadsheet. Assume that the following constitute the fixed and vari- able costs for the upcoming year Fixed Costs for...
-
Calculate the equilibrium constant Kc at 25oC from the free-energy change for the following reaction: See Appendix C for data. Zn(s) +2Ag (a)Zn2 (a) Ag(s)
-
Calculate the equilibrium constant at 25C and at 100C for each of the following reactions, using data available in Appendix 2A: (a) 2 CuO (s) 2 Cu(s) + O(g) (b) CH4(g) + H(g) CH6(g)
-
Use the data in the table to complete the following. (a) Make a scatterplot of the data. Estimate a value for b so that f(x) = 0.0002x b models the data. (b) Check the accuracy of f(x). (c) The moon...
-
Repeat Problem 66 for the resistor network shown in Figure. 12 S2
-
Let f: Rn R. For x Rn, a. Show that Deif (a) = Dif (a).. b. Show that Dtxf (a) = Dxf(a).. c. If f is differentiable at , show that Dxf(a) = Df(a)(x) (a) and therefore Dx + yf(a) = Dxf (a) + Dyf (a)..
-
Explain intraperiod tax allocation.
-
Using the data in E prepare a statement of cost of goods manufactured for the month ended January 31, 2016, assuming that no further work was done in Assembly during January. In E, AAA Appliances...
-
A taxpayer will be ineligible for the earned income credit if he or she has disqualified investment income of more than $3,500 in 2018. Disqualified income includes all the following except Group of...
-
How might one employee coach or train another employee by use of Twitter and text messaging?
-
Which of the following mixtures would you expect to show a positive deviation, a negative deviation, or no deviation (that is, form an ideal solution) from Raoults law? Explain your conclusion. (a)...
-
Calculate the osmotic pressure at 20C of each of the following solutions, assuming complete dissociation for any ionic solutes: (a) 0.050 m C 12 H 22 O 11 (aq); (b) 0.0010 m NaCl(aq); (c) A saturated...
-
Consider the following idealized PES spectrum for carbon: Explain the location and relative intensities of the various peaks. Relative number of electrons 100 10 Energy (MJ/mol) 1 0.1
-
Description: duff owes relatives $13,000 for college loans. find the required quarterly payment into a sinking fund if duff pays off the loan in 3 years and the interest rate is 8% per year...
-
1 3 , 9 5 0 ) Repairs and Maintenance ( $ 2 , 8 5 0 ) Utilities Expense ( $ 8 8 0 ) Operating Income $ 1 0 , 2 4 2 Other Income - Gain on Sale $ 3 0 0 Interest Expense ( $ 2 5 0 ) Earnings Before...
-
Description: The company currently has outstanding a bond with a 5.5 percent coupon rate and another bond with a 3.5 percent coupon rate. The firm has been informed by its investment banker that...
-
Find the equation of line joining the points (4, -3) and (-2, 7).
-
Calculate the work of reversible expansion of 1 mole of ideal gas at 25 degree celsius from 10 L to 20 L.
-
For the system of manometers shown in Figure P2.4. 11, determine the differential reading h. Two different manometry fluids are being used with different specific gravities. Water A Water B 46 cm 20...
-
Describe the Operations (+,,*,/) that can cause negligible addition (NA), error magnification (EM), or subtractive cancellation (SC) in calculating ?((x^2)+1) - x . Give the range of where they might...
-
The mechanism of the following transformation involves a carbocation intermediate that rearranges in a way that we have not yet seen. Rather than occurring via a methyl shift or a hydride shift, a...
-
An ideal gas is expanded adiabatically into a vacuum. Decide which of q, w, U and H is positive, negative, or zero.
-
Because U is a state function, (/V (U/T) V ) T = (/T (U/V) T ) V . Using this relationship, show that (CV/V) T = 0 for an ideal gas.
-
Need help filling out these tax forms. Not sure how to do 1040 page 2 or schedule 3. I think I have schedule 1 right but need help with the itemized deductions for 1040 page 1 Required information...
-
Question:What should Airbus and Boing have learned from IBERIA case? What changed in the industry when Boing decided to develop Dreamliner in 2003?( Read the following case and ppt) Airline Route...
-
Which of the following needs to be always assessed when you are evaluating the literature you have obtained for your research? O All of the above O Sufficiency Value O Relevance Several approaches...

Study smarter with the SolutionInn App


