Consider the following idealized PES spectrum for carbon: Explain the location and relative intensities of the various
Question:
Consider the following idealized PES spectrum for carbon:
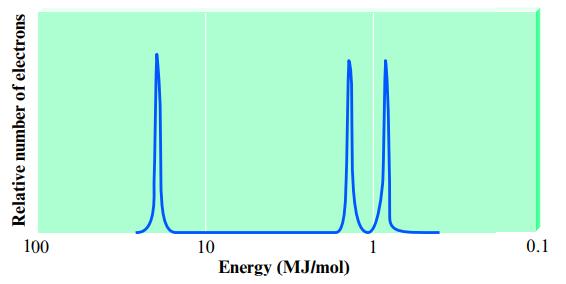
Explain the location and relative intensities of the various peaks.
Transcribed Image Text:
Relative number of electrons 100 10 Energy (MJ/mol) 1 0.1
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 83% (6 reviews)
Carbon has an atomic number of 6 The electronic setup of it is Orbitals come in three main variet...View the full answer

Answered By

Aketch Cindy Sunday
I am a certified tutor with over two years of experience tutoring . I have a passion for helping students learn and grow, and I firmly believe that every student has the potential to be successful. I have a wide range of experience working with students of all ages and abilities, and I am confident that I can help students succeed in school.
I have experience working with students who have a wide range of abilities. I have also worked with gifted and talented students, and I am familiar with a variety of enrichment and acceleration strategies.
I am a patient and supportive tutor who is dedicated to helping my students reach their full potential. Thank you for your time and consideration.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry
ISBN: 9781305957404
10th Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Question Posted:
Students also viewed these Engineering questions
-
Consider the following idealized PES spectrum for potassium: Explain the location and relative intensities of the various peaks. Relative number of electrons 1000 100 10 Energy (MJ/mol) 0.1
-
Consider the following idealized PES spectrum for an element: What is the identity of the element? Explain the relative positions of the various peaks. Relative number of electrons 1000 100 t 1 10...
-
Consider the following idealized PES spectrum for an element: What is the identity of the element? Relative number of electrons 1000 100 10 Energy (MJ/mol) 0.1
-
Hatch plc has two classes of share capital outstanding: 8%, 20 par preference and 5 par ordinary. At December 31, 2021, the following accounts were included in equity. Share CapitalPreference,...
-
For a new product, sales volume in the first year is estimated to be 80,000 units and is projected to grow at a rate of 4% per year. The selling price is $ 12 and will increase by $ 0.50 each year....
-
1. Summarize the process Mariam Yeseyan used in approaching women about loan opportunities through Aregak. 2. Compare opportunities for funding for women entrepreneurs through the commercial banking...
-
C&H Ski Club recently borrowed money and agrees to pay it back with a series of six annual payments of $5,000 each. C&H subsequently borrows more money and agrees to pay it back with a series of four...
-
Forey, Inc., competes against many other firms in a highly competitive industry. Over the last decade, several firms have entered this industry and, as a consequence, Forey is earning a return on...
-
On June 1, 2016, Brown Company, a new firm, paid $7,000 rent in advance for a seven-month period. The $7,000 was debited to the Prepaid Rent account. 2. On June 1, 2016, the firm bought supplies for...
-
Two children are randomly selected from a group of five boys and seven girls. Determine which is more likely to be selected: a. Two boys or two girls? b. The two youngest girls or the two oldest boys?
-
Give a possible set of values of the four quantum numbers for all the electrons in a boron atom and a nitrogen atom if each is in the ground state.
-
How many 4d electrons would be predicted in the ground state for the following elements? a. Zirconium b. Cadmium c. Iridium d. Iron
-
Sherry's utility is \(U_{S}\) and her income is \(Y_{S}\). Marsha's utility is \(U_{M}\) and her income is \(Y_{M}\). Suppose it is the case that: \(U_{S}=100 Y_{S}^{1 / 2}\), and \(U_{M}=100...
-
1. The KYM company wants to invest $ 523,000 pesos in the bank that guarantees a simple interest rate of 3.32% quarterly. If the company is considering the 8-month investment. What amount will you...
-
3. (5 points) The uncertainty principle limits our ability to determine simultaneously the position and momentum of a particle. (a) Why were classical physicists unaware of the limitations that this...
-
CASE STUDY Patient Name Valarie Ramirez Attending Paul F. Buckwalter, MD PATIENT INFORMATION DOB 08/04/1986 Allergies MAN 00-AA-006 Penicillin Other Information Past HX: AB x1 Valarie Ramirez arrives...
-
Petesy Corporation is preparing its Master Budget for 2019. Budget information is as follows: SalesProduction CostOperating Expenses 20191 st Quarter P280,000P192,000P64,000 2 nd Quarter 320,000...
-
A steady flow of 20 m3/s of moist air at TDB = 35iC, TWB = 25iC, 100 kPa (state 1) is dehumidified by first cooling it and condensing out moisture (state 2), then reheating it to 20iC and 50% R.H....
-
Anthony and Philip Conway founded and operated Rochester Medical Corporation (RMC), a publicly traded medical-device company. C.R. Bard, Inc., (Bard) offered to purchase RMC at a very attractive...
-
(a) Use integration by parts to show that (b) If f and g are inverse functions and f' is continuous, prove that (c) In the case where f and t are positive functions and b > a > 0, draw a diagram to...
-
How would the energy versus dihedral angle plot for 2-methylpropane (isobutene) differs from that for propane?
-
Draw an energy versus dihedral angle plot for the conformations of 2, 3-dirnethylbutane about the C-2C-3 bond.
-
Discuss the geometry and the types of strain present in these compounds: (a) Cyclopropane (b) Cyclobutene (c) Cyclopentane (d) Cyclohexane (e) Cyclodecane
-
nformation pertaining to Noskey Corporation s sales revenue follows: November 2 0 2 1 ( Actual ) December 2 0 2 1 ( Budgeted ) January 2 0 2 2 ( Budgeted ) Cash sales $ 1 0 5 , 0 0 0 $ 1 1 5 , 0 0 0...
-
The management team of Netflix maintains a stable dividend using the Lintner model: Dt+1 = Dt + EPS Target Payout Where Dt (Dt+1) = dividend in the current period t (the next period t + 1) EPSt =...
-
#1 #2 hapter 50 10 D Werences lav Help Required information [The following information applies to the questions displayed below) Archer Company is a wholesaler of custom-built air-conditioning units...

Study smarter with the SolutionInn App


