Consider the following idealized PES spectrum for an element: What is the identity of the element? Relative
Question:
Consider the following idealized PES spectrum for an element:
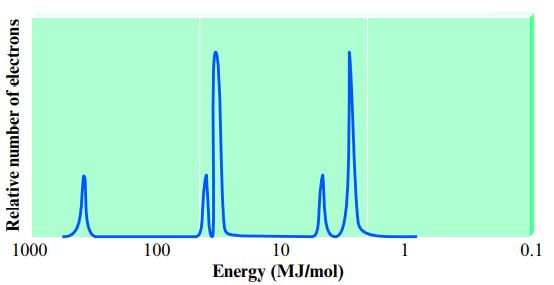
What is the identity of the element?
Transcribed Image Text:
Relative number of electrons 1000 100 10 Energy (MJ/mol) 0.1
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (9 reviews)
The elements name PES spectrum photo electron spectroscopy is a met...View the full answer

Answered By

Aketch Cindy Sunday
I am a certified tutor with over two years of experience tutoring . I have a passion for helping students learn and grow, and I firmly believe that every student has the potential to be successful. I have a wide range of experience working with students of all ages and abilities, and I am confident that I can help students succeed in school.
I have experience working with students who have a wide range of abilities. I have also worked with gifted and talented students, and I am familiar with a variety of enrichment and acceleration strategies.
I am a patient and supportive tutor who is dedicated to helping my students reach their full potential. Thank you for your time and consideration.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry
ISBN: 9781305957404
10th Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Question Posted:
Students also viewed these Engineering questions
-
Consider the following idealized PES spectrum for an element: What is the identity of the element? Explain the relative positions of the various peaks. Relative number of electrons 1000 100 t 1 10...
-
Consider the following idealized PES spectrum for potassium: Explain the location and relative intensities of the various peaks. Relative number of electrons 1000 100 10 Energy (MJ/mol) 0.1
-
Consider the following idealized PES spectrum for carbon: Explain the location and relative intensities of the various peaks. Relative number of electrons 100 10 Energy (MJ/mol) 1 0.1
-
Laura Leasing SA signs an agreement on January 1, 2022, to lease equipment to Plote AG. The following information relates to this agreement. 1. The term of the non-cancelable lease is 3 years with no...
-
The Radio Shop sells two popular models of portable sport radios, model A and model B. The sales of these products are not independent of each other (in economics, we call these substitutable...
-
1. What is strategic leadership? 2. What would constitute key strategic leadership actions? What are the key elements of a Balanced Scorecard? 3. How has Cheung Yan seen success as a strategic...
-
Otto Co. borrows money on April 30, 2008, by promising to make four payments of $13,000 each on November 1, 2008; May 1, 2009; November 1, 2009; and May 1, 2010. 1 How much money is Otto able to...
-
Restate the following question component of the issue in a persuasive manner. A. "should the evidence be suppressed when . . . ?" In the case, the police failed to obtain a search warrant prior to...
-
Complete the table below for the missing variances Click the icon to view the table) Calculate the variances and identity whether the variance in favorable (F) or unfavorable (U). (b) Total Direct...
-
Recall that [[x] is the greatest integer function. For R of (a) (b) (x [y]) dA IR
-
Give a possible set of values of the four quantum numbers for all the electrons in a boron atom and a nitrogen atom if each is in the ground state.
-
How many 4d electrons would be predicted in the ground state for the following elements? a. Zirconium b. Cadmium c. Iridium d. Iron
-
A team of researchers has obtained a dinosaur bone (Tyrannosaurus rex) and has attempted to extract ancient DNA from it. Using primers to the 12S rRNA mitochondrial gene, they have used PCR and...
-
Question 37 Plantito Inc., produces potted plants. For next year, Pietro predicts that 45,000 units will be produced, with the following total costs: Direct materials Direct labor ? 80,000 Variable...
-
When you are to design a data transmission system, you have two key considerations to work with: data rate and distance, with emphasis placed on achieving the highest data rates over the longest...
-
How much work does a supermarket checkout attendant do on a can of soup he pushes 0.600 m horizontally with a force of 5.00 N? Express your answer in joules and kilocalories. 3 . (a) Calculate the...
-
Suppose in its income statement for the year ended June 30, 2022, The Clorox Company reported the following condensed data (dollars in millions). Salaries and wages expenses$460 Research and...
-
Consider the extensive form game show in the figure below. How many strategies does Player 2 have in this game? (2,2,1) b (2,4,2) 03 by 03 02 dz (4.2,0) (2.0.2) (0.3.4) (3,5,3) (3,1,2)
-
Licensed taxicab drivers in Boston brought an action against cab companies, alleging that they were misclassified by the companies as independent contractors. The taxicab drivers alleged that they...
-
Find a polar equation for the curve represented by the given Cartesian equation. 4y 2 = x
-
Explain why the axial strain energies for the C N group and the C CH group are much smaller than that for the CH 3 group.
-
Draw the chair conformations of 1, 1, 3trimethyl cyclohexane. Which conformation is more stable? Why is it not possible on the basis of the material in this chapter to determine the exact energy...
-
Draw the cis and trans stereo isomers of this compound and explain their relative stabilities. OH CH
-
Long-term liabilities are shown in two places in the business firm's balance sheet depending upon when the long-term liabilities are scheduled for payment. True False
-
Julio is single with 1 withholding allowance. He earned $1,025.00 during the most recent semimonthly pay period. He needs to decide between contributing 3% and $30 to his 401(k) plan. If he chooses...
-
Acquirer firm plans to launch a takeover of Target firm. The manager of Acquirer indicates that the deal will increase the free cash flow of the combined business by $13.6m per year forever. The beta...

Study smarter with the SolutionInn App


