Consider the following idealized PES spectrum for potassium: Explain the location and relative intensities of the various
Question:
Consider the following idealized PES spectrum for potassium:
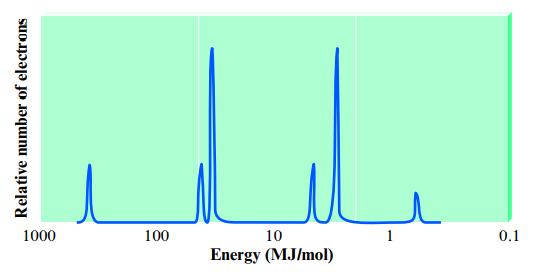
Explain the location and relative intensities of the various peaks.
Transcribed Image Text:
Relative number of electrons 1000 100 10 Energy (MJ/mol) 0.1
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 71% (7 reviews)
Potassium has an atomic number of 19 The electronic setup of it is Orbitals co...View the full answer

Answered By

Aketch Cindy Sunday
I am a certified tutor with over two years of experience tutoring . I have a passion for helping students learn and grow, and I firmly believe that every student has the potential to be successful. I have a wide range of experience working with students of all ages and abilities, and I am confident that I can help students succeed in school.
I have experience working with students who have a wide range of abilities. I have also worked with gifted and talented students, and I am familiar with a variety of enrichment and acceleration strategies.
I am a patient and supportive tutor who is dedicated to helping my students reach their full potential. Thank you for your time and consideration.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry
ISBN: 9781305957404
10th Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Question Posted:
Students also viewed these Engineering questions
-
Consider the following idealized PES spectrum for carbon: Explain the location and relative intensities of the various peaks. Relative number of electrons 100 10 Energy (MJ/mol) 1 0.1
-
Consider the following idealized PES spectrum for an element: What is the identity of the element? Relative number of electrons 1000 100 10 Energy (MJ/mol) 0.1
-
Consider the following idealized PES spectrum for an element: What is the identity of the element? Explain the relative positions of the various peaks. Relative number of electrons 1000 100 t 1 10...
-
Pew Research published survey results from two random samples. Both samples were asked, Have you listened to an audio book in the last year? The results are shown in the table below. a. Find and...
-
"Part 1: The Performance Lawn Equipment database contains data needed to develop a pro forma income statement. Dealers selling PLE products all receive 18% of sales revenue for their part of doing...
-
Why are materials that are good thermal conductors also good electrical conductors? What kinds of problems does this pose for the design of appliances such as clothes irons and electric heaters? Are...
-
Find the amount of money that can be borrowed today with each of the following separate debt agreements a through f: Single Future Number Interest Case Payment of Periods Rate a. . . . . . . . ....
-
Refer to the financial statements of American Eagle Outfitters given in Appendix B at the end of this book. At the bottom of each statement, the company warns readers to "Refer to Notes to...
-
Which of the following individuals would normally be included on the budget committee? 1. Company president 2. Sales manager O Neither 1 nor 2 O Both 1 and 2 O 1 only O 2 only Save for Later
-
Please assume figures are USD currency unless otherwise specified and provide all answers in USD. 1 You've been given the following information: - Market/reference prices for a variety of metals and...
-
Give a possible set of values of the four quantum numbers for all the electrons in a boron atom and a nitrogen atom if each is in the ground state.
-
How many 4d electrons would be predicted in the ground state for the following elements? a. Zirconium b. Cadmium c. Iridium d. Iron
-
Answer the following questions about force on a moving charge. A particle with a positive unit charge (q = 1) enters a constant magnetic field B = i + j with a velocity v = 20k. Find the magnitude...
-
The current rate of interest on S-T Treasury Bills = 10%, intermediate term Gov. Bonds = 11%, Lt- Gov. Bonds = 12%, AA rate Corp. Bonds = 13.5% and the rate of inflation is 5%. Holding-period returns...
-
Prepare Income Statement(absorption costing) for the second, third and fourth month. SALES (SP X unit sold) INCOME STATEMENT FORMAT (ABSORPTION COSTING) XXX Less: Cost of Goodsold VARIABLE COST (VC...
-
The following shows the distribution of final exam scores in a large introductory psychology class. The proportion under the curve is given for two segments (short answers-no calculations required)....
-
How much overhead was included in the cost of Job #461 at the beginning of January? * (1 Point). BREAD Co. uses a job order costing system. At the beginning of January, the company had 2 jobs in...
-
3. (3pt.) A state of a physical system is just a description of the system at an instant in time in terms of its properties. In classical mechanics, states are represented by points (in phase space)....
-
An employee who suffers from fibromyalgia and degenerative disc and cervical disease worked as a Pulaski County juvenile detention officer from November 24, 2001, to May 21, 2013. Beginning in 2008,...
-
A survey of 70 college freshmen asked whether students planned to take biology, chemistry, or physics during their first year. Use the diagram to answer each question. How many of the surveyed...
-
Draw all the cis-trans isomers for these compounds: (a) CH 3 CH = CHCH = CHCH 2 CH 3 (b) CH 3 CH = CHCH = CHCH 3 (c) CH 3 CH = CHCH = CH 2
-
Which of these groups has the higher priority? Br b) -C=N or -CH,CH2 a) -C=CH or CH3 CH3 c) -C=CH2 or -C-CH3 CH3 -C-CH3 d) -C=CH or CH3
-
Assign the configurations of these compounds as Z or E: 3 CH,CH2CH3 C=C CH,Br C=C HOCH,CH2 b) a) H,NCH, CH,CHCH3 CH,CH CH3 H3 - HO- Ph c) d)
-
Given the following financial data for the Smith Corporation, calculate the length of the firm's operating cycle (OC). Sales $2,610,000 Cost of Good Sold $2,088,000 Inventory $ 278,400 Accounts...
-
The predetermined overhead rate is usually calculated Group of answer choices At the end of each year At the beginning of each month At the beginning of the year At the end of the month
-
ajax county collects property taxes for the cities within the county, Ajax county collected 1000 from citizens in Beatty city that belong to Beatty city what would be the appropriate entries for ajax...

Study smarter with the SolutionInn App


