Draw an orbital energy-level diagram (like those in Figs. 9D.3 and 9D.5) showing the configuration of d-electrons
Question:
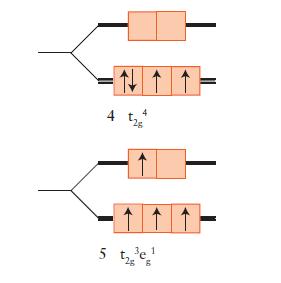
Draw an orbital energy-level diagram (like those in Figs. 9D.3 and 9D.5) showing the configuration of d-electrons on the metal ion in each of the following complexes:
(a) [Co(NH3)6]3+;
(b) [NiCl4]2– (tetrahedral);
(c) [Fe(OH2)6]3+;
(d) [Fe(CN)6]3–.
Predict the number of unpaired electrons for each complex.
FIGURE 9D.3
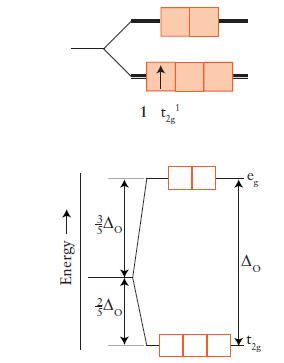
FIGURE 9D.5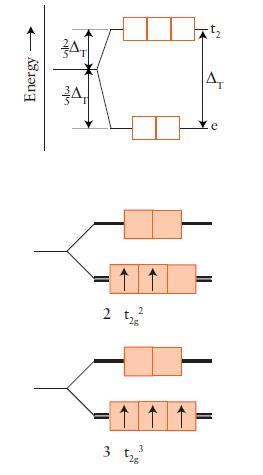
Transcribed Image Text:
دار Energy → داری 1
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (2 reviews)
a octahedral strongfield ligand 6 e no unpaired electrons 31...View the full answer

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Draw an orbital energy-level diagram (like those in Figs.9D.3 and 9D.5) showing the configuration of d-electrons on the metal ion in each of the following complexes: (a) [Zn(OH 2 ) 6 ] 2+ ; (b) [CoCl...
-
Draw a molecular orbital energy level diagram for each of the following species: He+2, HHe, He2+. Compare their relative stabilities in terms of bond orders. (Treat HHe as a diatomic molecule with...
-
You are working on a free-form Packet Tracer challenge activity as seen in Figure 1, you have been given the London Railways network.' The purpose of this EMA question is to build upon each of the...
-
Describes 7shifts' pioneering role in revolutionizing restaurant management through cloud-based solutions.
-
In the solution to the bounded buffer problem (Figure), consider the ordering of the first two P operations in the producer and the consumer. Suppose the order of the p(full) and the p(mutex)...
-
Ms. Smith is retired and depends on her investments for retirement income. Mr. Jones is a young executive who wants to save for the future. They are both stockholders in Airbus, which is investing...
-
The prism in Figure P24.32 is symmetric (i.e., its face forms an isosceles triangle). If the ray showed is perpendicular to the bottom surface of the prism and just barely undergoes total internal...
-
Hitachi, Ltd., reports total revenues of 9,041,071 million for its fiscal year ending March 31, 2013, and its March 31, 2013, unadjusted trial balance reports a debit balance for trade receivables...
-
Problem 19-17 Full-Capacity Sales (LO4, CFA9) Thorpe Mfg., Inc., is currently operating at only 77 percent of fixed asset capacity. Current sales are $730,000. How fast can sales grow before any new...
-
New pharmaceuticals must be enantiomerically pure for use in human medicine. If you work for a large biotechnology company, you will need to be able to recognize chiral sites in complex molecules....
-
Use the information in Table 9C.1 to write the formula for each of the following coordination compounds: (a) Triamminediaquabromidocobalt(II) hydroxide (b) Dichloridobisethylenediaminecobalt(III)...
-
In Problems find the pivot element, identify the entering and exiling variables, and perform one pivot operation. 1 6 1 0 0 36 -1 2 0 010
-
What should be the equivalent units of production for (1) Dept M and (2) Dept. P? Can you please show the solutions and answer. Thanks Problem 1 Lee Gon Mfg. Co has its product processed in two...
-
Moullierat Mfg. is considering a rights offer. The company has determined that the ex-rights price will be $95. The current price is $102 per share, and there are 24 million shares outstanding. The...
-
This question involves hypothesis testing. The following numbers will help you answer these questions. The random variable Z ~N(0, 1) is standard normal. P(Z >1.28).1 P(Z1.65) .05 P(Z1.96) .025 P(Z...
-
Human service organizations require strong and effective leadership. Understanding what qualities make up an effective leader and how these qualities can be cultivated is of critical importance for...
-
18. What is the name of the heat treatment performed on a cold worked sample? 19. What is the percent coldwork of a sample with an initial thickness of 11mm and a final thickness of 7mm? 20. Which...
-
Use the data in TWOYEAR.RAW for this exercise. (i) The variable stotal is a standardized test variable, which can act as a proxy variable for unobserved ability. Find the sample mean and standard...
-
Pappa's Appliances uses the periodic inventory system. Details regarding the inventory of appliances at January 1, purchases invoices during the year, and the inventory count at December 31 are...
-
Consider the energy-level diagrams depicted in the text. a. At what temperature will the probability of occupying the second-energy level be 0.15 for the states depicted in part (a) of the figure? b....
-
Consider the energy-level diagrams, modified from Problem P30.9 by the addition of another excited state with energy of 600. cm 1 . a. At what temperature will the probability of occupying the second...
-
Consider the following sets of populations for four equally spaced energy levels: a. Demonstrate that the sets have the same energy. b. Determine which of the sets is the most probable. c. For the...
-
A zero-coupon bond bears a higher interest rate risk than a coupon-paying bond, given other bond characteristics are equal. True False
-
Company management decided to restructure its balance sheet. Current long term debt of 8 mio euros will be increased to 20 mio euros. Interest for the debt is 4%. Borrowed 12 mio euros will used to...
-
Susan loans 10000 to Jim. Jim repays the loan with yearly instalments at the end of each year. Interest is expected to be 5% the first five years, and 10% the last five years. Calculate the...

Study smarter with the SolutionInn App


