Use the information in Table 9C.1 to write the formula for each of the following coordination compounds:
Question:
Use the information in Table 9C.1 to write the formula for each of the following coordination compounds:
(a) Triamminediaquabromidocobalt(II) hydroxide
(b) Dichloridobisethylenediaminecobalt(III) bromide
(c) Sodium triamminetrichloridonickelate(II)
(d) Barium tris(oxalato)ferrate(III)
(e) Diaquadichloridoplatinum(IV) iodide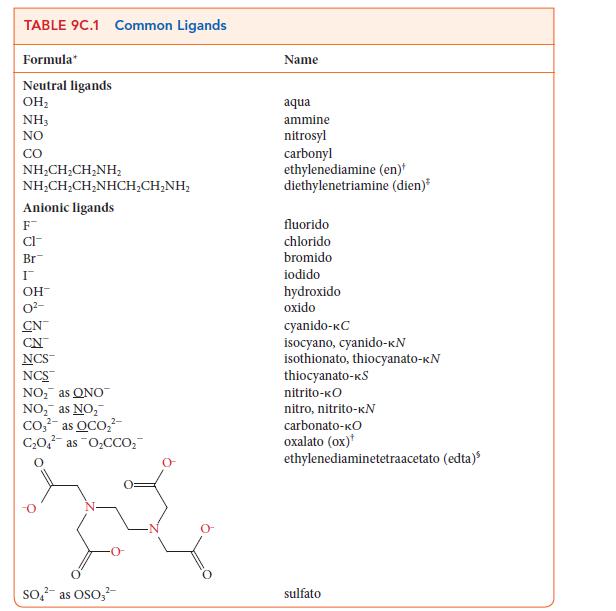
Transcribed Image Text:
TABLE 9C.1 Common Ligands Formula Neutral ligands OH₂ NH3 NO CO NHẠCH,CH_NH, NHẠCH,CH,NHCH,CHÍNH, Anionic ligands F CI- Br I OH- CN NCS™ NCS™ NO₂ as ONO NO₂ as NO₂ Co₂² as OCO₂² C₂O4 as O₂CCO₂ 2- SO² as OSO3² Name aqua ammine nitrosyl carbonyl ethylenediamine (en) diethylenetriamine (dien)* fluorido chlorido bromido iodido hydroxido oxido cyanido-KC isocyano, cyanido-kN isothionato, thiocyanato-KN thiocyanato-kS nitrito-KO nitro, nitrito-KN carbonato-kO oxalato (ox) ethylenediaminetetraacetato (edta) sulfato
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
Solution a CoNH3...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Write the formula for each of the following compounds: a. Isobutyl chloride b. isopropyl bromide c. 2-chlorobutane d. tert-butyl iodide e. Propyl fluoride f. general formula for an alkyl bromide
-
Write the formula for each of the following compounds, being sure to use brackets to indicate the coordination sphere: (a) Hexaamminechromium (III) nitrate (b) Tetraamminecarbonatocobalt (III)...
-
Write a structural formula for each of the following compounds a. Sec-butyl tert-butyl ether b. Isoheptyl alcohol c. Sec-butylamine d. Neopentyl bromide e. 1,1-dimethylcyclohexane f....
-
What is true about business cycles? Multiple select question. They vary in duration and intensity. They follow an identical pattern. They are made up of alternating rises and declines. "Ups" are...
-
Dijkstra posed each of the following solutions as a potential software solution to the critical section problem and then explained why they failed [Dijkstra, 1968]. Provide your explanation about why...
-
Why is a reputation for honesty and fair business practice important to the financial value of the corporation?
-
A rectangular slab of glass has an index of refraction n = 1.80. Light is incident at 45, striking the upper surface very close to the right edge as shown in Figure P24.33, and is refracted as it...
-
The Board Shop, owned by Andrew John, sells skateboards in the summer and snowboards in the winter. The shop has an August 31 fiscal year end and uses a perpetual inventory system. On August 1, 2017,...
-
TASK 5: PUT IN CITATION Explain two (2) similarities and three (3) differences between IFRS and US GAAP with respect to accounting for inventories. PUT REFERENCES AS WELL
-
Draw an orbital energy-level diagram (like those in Figs. 9D.3 and 9D.5) showing the configuration of d-electrons on the metal ion in each of the following complexes: (a) [Co(NH 3 ) 6 ] 3+ ; (b)...
-
You are an engineer studying materials for computer hard-drive fabrication and need to predict the magnetic properties of the iron complexes you are investigating. Compare the magnetic properties of...
-
Sean Moon is president, secretary, treasurer, sole director, and sole shareholder of Streetz, an S corporation real estate company. He manages all aspects of the companys operations, and he is the...
-
Pulleys C and D in Figure are fastened together. Weights A and B are supported by ropes wound around the pulleys as shown. The radius for pulley C is 187 mm and the radius for pulley D is 138 mm. If...
-
As a leader what are some of the thoughtful and creative ideas that you have implemented to motivate your team and increase job satisfaction?
-
A Chinese smartphone maker TECNO Ltd has provided you with a summary of its price and cost information for one of its product segments (tablets). It is based on 2018 income statement. Units produced...
-
The following projected financial data is available for the single product of Janis Ltd:- October November December Sales (unit) 50,000 65,000 65,000 Production (unit) 70,000 60,000 50,000 Opening...
-
I would appreciate freehand sketches for the top, side views, and front views. D C 6 50 B 2.75 A a 5 4 3 2 1 .45 UNLESS OTHERWISE SPECIFIED: DIMENSIONS ARE IN MILLIMETERS SURFACE FINISH: TOLERANCES:...
-
Use the data in LOANAPP.RAW for this exercise. (i) How many observations have obrat > 40, that is, other debt obligations more than 40% of total income? (ii) Reestimate the model in part (iii) of...
-
Frontland Advertising creates, plans, and handles advertising campaigns in a three-state area. Recently, Frontland had to replace an inexperienced office worker in charge of bookkeeping because of...
-
A set of 13 particles occupies states with energies of 0, 100, and 200 cm 1 . Calculate the total energy and number of microstates for the following configurations of energy: a. a 0 = 8, a 1 = 5, and...
-
For a set of non-degenerate levels with energy /k = 0,100, and 200. K, calculate the probability of occupying each state when T = 50, 500, and 5000.K. As the temperature continues to increase, the...
-
For two non-degenerate energy levels separated by an amount of energy /k = 500.K, at what temperature will the population in the higher-energy state be 1/2 that of the lower-energy state? What...
-
Jennifer purchased a home for $1,000,000 in 2016. She paid $200,000 cash and borrowed the remaining $800,000. This is Jennifer's only residence. Assume that in year 2024, when the home had...
-
business plan describing company with strengths and weaknesses. Any gaps in plan. Recommendations for improvement of the plan.
-
You wish to buy a car today for $35,000. You plan to put 10% down and finance the rest at 5.20% p.a. for six years. You will make equal monthly payments of $_______.

Study smarter with the SolutionInn App


