Draw an orbital energy-level diagram (like those in Figs.9D.3 and 9D.5) showing the configuration of d-electrons on
Question:
Draw an orbital energy-level diagram (like those in Figs.9D.3 and 9D.5) showing the configuration of d-electrons on the metal ion in each of the following complexes:
(a) [Zn(OH2)6]2+;
(b) [CoCl4]2– (tetrahedral);
(c) [Co(CN)6]3–;
(d) [CoF6]3–.
Predict the number of unpaired electrons for each complex.
FIGURE 9D.3
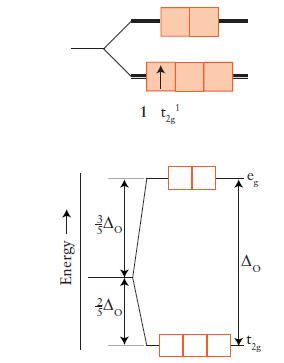
FIGURE 9D.5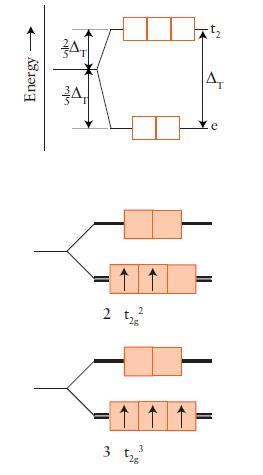
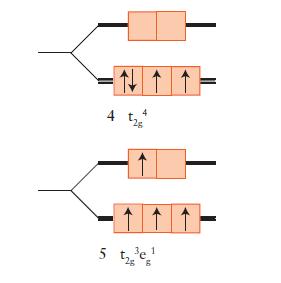
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:





