EDTA is used as a complex agent in chemical analysis. Solutions of EDTA, usually containing the disodium
Question:
EDTA is used as a complex agent in chemical analysis. Solutions of EDTA, usually containing the disodium salt Na2H2EDTA, are also used to treat heavy metal poisoning. The equilibrium constant for the following reaction is 6.7 × 10-1: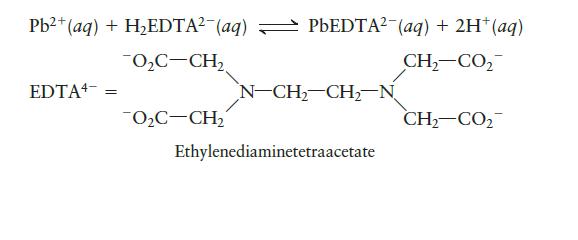
Transcribed Image Text:
Pb²+ (aq) + H₂EDTA² (aq) PbEDTA² (aq) + 2H+ (aq) 0₂C-CH₂ CH,CO, CH,CO2 EDTA = O₂C-CH₂ = N-CH₂-CH₂-N Ethylenediaminetetraacetate
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (8 reviews)

Answered By

Charles mwangi
I am a postgraduate in chemistry (Industrial chemistry with management),with writing experience for more than 3 years.I have specialized in content development,questions,term papers and assignments.Majoring in chemistry,information science,management,human resource management,accounting,business law,marketing,psychology,excl expert ,education and engineering.I have tutored in other different platforms where my DNA includes three key aspects i.e,quality papers,timely and free from any academic malpractices.I frequently engage clients in each and every step to ensure quality service delivery.This is to ensure sustainability of the tutoring aspects as well as the credibility of the platform.
4.30+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Start off with a brief introduction describing the main concepts of the lab including both invasive species information and successional types and urban ecology. After that, answer the following...
-
The equilibrium constant for the following reaction is 1.0 Ã 10-3: Cr'. (aq) + H2EDTA2-(aq)--CrEDT A-(aq) + 2H' (aq) CH2-CO2 02GH CH EDTA N-CH,--CH2- O2C-CH2 CHy-CO2-...
-
A wooden board AB, which is used as a temporary prop to support a small roof, exerts at point A of the roof a 228-N force directed along BA. Determine the moment about C of that force. 0.12 m D. 18m
-
Gregorian Company has recently issued bonds that are convertible into stock at the bondholders request. The interest rate on the bonds is ridiculously low because it is expected that most holders...
-
From calls made with randomly generated telephone numbers, 1012 respondents are asked if they rent or own their residences. Identify the sampling technique used, and discuss potential sources of bias...
-
Could the list of gods example from 17.10.1 have been written using a singly-linked list; that is, could we have left the prev member out of Link? Why might we want to do that? For what kind of...
-
What is the main technique used for validating scope? Give an example of scope validation on a project. LO.1
-
Following are income statements for Hossa Corporation for 2017 and 2016. Percentage of sales amounts are also shown for each operating expense item. Hossas income tax rate was 38% in 2016 and 40% in...
-
1. Peter transfers a piece of land to Partnership PAT for a partnership interest, The land had an adjusted basis in Peters hands of $25 and a fair market value of $100. Peter bought the land 3 years...
-
A computer center has three printers, A, B and C, which print at different speeds. Programs are routed to the first available printer. The probability that the program to printers A, b and C are 0.6,...
-
A series of chemicals were added to some AgNO 3 (aq). NaCl(aq) was added first to the silver nitrate solution with the end result shown below in test tube 1, NH 3 (aq) was then added with the end...
-
What is the hybridization of the underlined nitrogen atom in each of the following molecules or ions? a. NO - b. N 2 O 3 (O 2 NNO) c. NO 2 - d. N 2
-
Which of the following writes the contents of the city and state variables to an output file named address.txt? The file is associated with the outFile object. a. address.txt < < city < < state < <...
-
In the global discourse on healthcare, the United States and England stand out as two contrasting models, each providing a distinct approach to addressing the challenges of cost , access, and...
-
2.A. Using the quotes below, answer the following questions. Exchange rate Bid Ask In New York, USD/EUR 1.2267 1.2875 In London, USD/GBP 1.6555 1.7334 2.A1. Calculate the EUR/GBP cross exchange...
-
Question 43 Part B Q1ii 20 points Save A a) A property is currently leased for $100,000 p.a. with fully recoverable outgoings. The lease has 3 years to run on the current (fixed) rent. The market...
-
Define HIPPA? What is the purpose of HIPPA? What are the 4 main rules of HIPPA?
-
Accounting for Inventories" Please respond to the following: As a Financial Accountant,determine the best type of income statement a retailer should use.Defend your suggestion. Analyze inventory...
-
Find all intervals where each product will at least break even. The cost to produce x units of baseball caps is C = 100x + 6000, while the revenue is R = 500x.
-
Nitrogen monoxide reacts with hydrogen as follows: 2NO(g)+ H2(g) N2O(g) + H2O(g) The rate law is [H2]/ t = k[NO]2[H2], where k is 1.10 107 L2/(mol2s) at 826oC. A vessel contains NO and H2 at...
-
Explain why the radial distribution function rather than the square of the magnitude of the wave function should be used to make a comparison with the shell model of the atom.
-
What is the difference between an angular and a radial node? How can you distinguish the two types of nodes in a contour diagram such as Figure 20.7? 20 10 -10 -20 -20 -10 10 20 20 10 -10 -20 -20 -10...
-
What is the minimum photon energy needed to ionize a hydrogen atom in the ground state?
-
Regarding Enron, this was a company that resulted in the creation of the Sarbanes-Oxley Act and many reforms to the accounting profession. Research the company and answer the following...
-
Clayton received a $140,000 distribution from his 401(k) account this year. Assuming Clayton's marginal tax rate is 25 percent, what is the total amount of tax and penalty Shauna will be required to...
-
Mass LLp developed software that helps farmers to plow their fiels in a mannyue sthat precvents erosion and maimizes the effoctiveness of irrigation. Suny dale paid a licesnsing fee of $23000 for a...

Study smarter with the SolutionInn App


