For the exercise in this topic, base your answers on the potentials listed in Table 6M.1 or
Question:
For the exercise in this topic, base your answers on the potentials listed in Table 6M.1 or Appendix 2B, with the exception of the reduction and oxidation of water at pH = 7: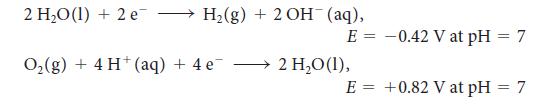 Suppose that 2.69 g of a silver salt (AgX) is dissolved in 550 mL of water. With a current of 3.5 A, 395.0 s was needed to plate out all the silver.
Suppose that 2.69 g of a silver salt (AgX) is dissolved in 550 mL of water. With a current of 3.5 A, 395.0 s was needed to plate out all the silver.
(a) What is the mass percentage of silver in the salt?
(b) What is the formula of the salt?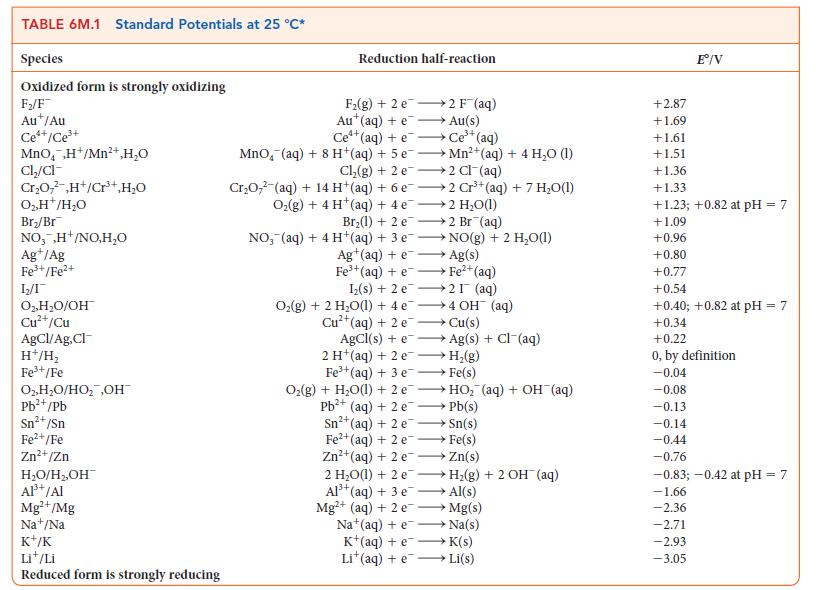
Transcribed Image Text:
2 H₂O(1) + 2 e → H₂(g) + 2 OH¯ (aq), E = O₂(g) + 4H+ (aq) + 4e¯ 2 H₂O (1), E = -0.42 V at pH = 7 +0.82 V at pH = 7
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (2 reviews)
a ...View the full answer

Answered By

Ali Khawaja
my expertise are as follows: financial accounting : - journal entries - financial statements including balance sheet, profit & loss account, cash flow statement & statement of changes in equity -consolidated statement of financial position. -ratio analysis -depreciation methods -accounting concepts -understanding and application of all international financial reporting standards (ifrs) -international accounting standards (ias) -etc business analysis : -business strategy -strategic choices -business processes -e-business -e-marketing -project management -finance -hrm financial management : -project appraisal -capital budgeting -net present value (npv) -internal rate of return (irr) -net present value(npv) -payback period -strategic position -strategic choices -information technology -project management -finance -human resource management auditing: -internal audit -external audit -substantive procedures -analytic procedures -designing and assessment of internal controls -developing the flow charts & data flow diagrams -audit reports -engagement letter -materiality economics: -micro -macro -game theory -econometric -mathematical application in economics -empirical macroeconomics -international trade -international political economy -monetary theory and policy -public economics ,business law, and all regarding commerce
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
For the exercise in this topic, base your answers on the potentials listed in Table 6M.1 or Appendix 2B, with the exception of the reduction and oxidation of water at pH = 7: A sample of manganese of...
-
For the exercise in this topic, base your answers on the potentials listed in Table 6M.1 or Appendix 2B, with the exception of the reduction and oxidation of water at pH = 7: (a) How much time is...
-
For the exercise in this topic, base your answers on the potentials listed in Table 6M.1 or Appendix 2B, with the exception of the reduction and oxidation of water at pH = 7: Thomas Edison was faced...
-
- The first stream is $450 per year for 9 years and begins 5 years from today - The second stream begins 7 years from today with the first cash flow being $400, and with each successive cash flow...
-
Strategic analysis of operating income. Halsey Company sells womens clothing. Halseys strategy is to offer a wide selection of clothes and excellent customer service and to charge a premium price....
-
A pilot flies horizontally at 1300km/h, at height h = 35 m above initially level ground. However, at time t = 0, the pilot begins to fly over ground sloping upward at angle θ = 4.3o (Figure)....
-
What do you see as the primary challenges to introducing a project management philosophy in most organizations? That is, why is it difficult to shift to a project-based approach in many companies?
-
The intangible assets section of Amato Corporation's balance sheet at December 31, 2017, is presented here. Patents ($60,000 cost less $6,000 amortization).........................$54,000 Copyrights...
-
, , , , , , ( ) , , , , Q )
-
Micro Technologies sells two products: X and Y. The selling price, variable cost, and sales mix for the products are presented in the table below. Product Unit Sales Price Unit Variable Cost...
-
Use the thermodynamic data in Appendix 2A to calculate the acidity constant of HF(aq). 2A THERMODYNAMIC DATA AT 25 C Inorganic Substances Substance Aluminum Al(s) Al+ (aq) AI(OH)3(s) AlO3(s) AICI,...
-
The pH of 0.50 m HBrO(aq) is 4.50. Calculate the change in pH when 5.10 g of sodium hypobromite is added to 100. mL of the solution. Ignore any change in volume.
-
The given numbers represent angle measure. Express the measure of each angle in degrees. 36.07
-
do you agree wih this approach to dismantling the toxic culture? explain
-
Movies When randomly selecting a speaking character in a movie, the probability of getting a female is 0.331 (based on data from "Inequality in 1200 Popular Films," by Smith, et al., Annenberg...
-
Steve Reese is a well-known interior designer in Fort Worth, Texas. He wants to start his own business and convinces Rob O'Donnell, a local merchant, to contribute the capital to form a partnership....
-
Exercise 6-10A (Algo) Double-declining-balance and units-of-production depreciation: gain or loss on disposal LO 6-3, 6-4, 6-5 Exact Photo Service purchased a new color printer at the beginning of...
-
Independent Events Again assume that when randomly selecting a speaking character in a movie, the probability of getting a female is 0.331, as in Exercise 1. If we want to find the probability of 20...
-
Compute the standard error of (1 - 2) for the following data: Sample 1 10 125 44.2 Sample 2 10 217 rt 28.7
-
A horizontal annulus with inside and outside diameters of 8 and 10 cm, respectively, contains liquid water. The inside and outside surfaces are maintained at 40 and 20oC, respectively. Calculate the...
-
Consider the reaction FeO(s) + CO(g) Fe(s) + CO 2 (g) for which K P is found to have the following values: a. Calculate ÎG o R , ÎS o R , and ÎHR???? for this reaction at...
-
If K P is independent of pressure, why does the degree of dissociation in the reaction Cl 2 (g) 2Cl(g) depend on pressure?
-
How does the total number of moles in the reaction system change as T increases? H 2 (g) + Cl 2 (g) 2HCl(g) at equilibrium. Assume ideal gas behavior.
-
A project will generate annual cash flows of $237,600 for each of the next three years, and a cash flow of $274,800 during the fourth year. The initial cost of the project is $749,600. What is the...
-
You want to invest annual amounts over the next 15 years. If your goal is to have $15,000 at the end of that time and if you can earn 8 percent on your invested funds, how much do you need to invest...
-
please explain thoroughly how to do in excel 1. Find the number of units to ship from each factory to each customer that minimizes total cost

Study smarter with the SolutionInn App


