Use the thermodynamic data in Appendix 2A to calculate the acidity constant of HF(aq). 2A THERMODYNAMIC DATA
Question:
Use the thermodynamic data in Appendix 2A to calculate the acidity constant of HF(aq).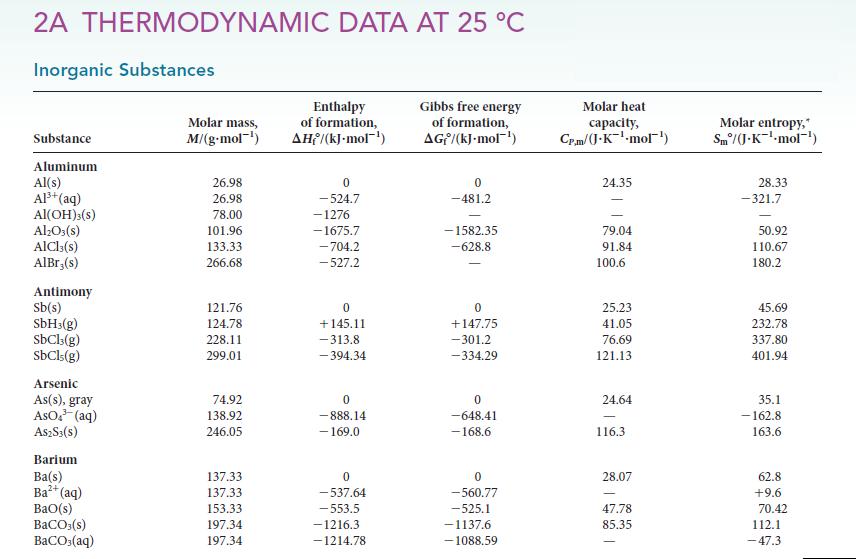
Transcribed Image Text:
2A THERMODYNAMIC DATA AT 25 °C Inorganic Substances Substance Aluminum Al(s) Al³+ (aq) AI(OH)3(s) Al₂O3(s) AICI, (s) AlBr,(s) Antimony Sb(s) SbH3(g) SbCl3(g) SbCls(g) Arsenic As(s), gray AsO4³ (aq) A$2S3(S) Barium Ba(s) Ba²+ (aq) BaO(s) BaCO3(s) BaCO3(aq) Molar mass, M/(g-mol-¹) 26.98 26.98 78.00 101.96 133.33 266.68 121.76 124.78 228.11 299.01 74.92 138.92 246.05 137.33 137.33 153.33 197.34 197.34 Enthalpy of formation, AH/(kJ.mol-¹) 0 -524.7 -1276 -1675.7 -704.2 -527.2 0 +145.11 -313.8 -394.34 0 -888.14 - 169.0 0 -537.64 -553.5 -1216.3 -1214.78 Gibbs free energy of formation, AG/(kJ-mol-¹) 0 -481.2 -1582.35 -628.8 0 +147,75 -301.2 -334.29 0 -648.41 -168.6 0 -560.77 -525.1 -1137.6 -1088.59 Molar heat capacity, Cr.m/(J-K-mol¹) 24.35 79.04 91.84 100.6 25.23 41.05 76.69 121.13 24.64 116.3 28.07 47.78 85.35 Molar entropy," Sm/(J.K¹-mol-¹) 28.33 -321.7 50.92 110.67 180.2 45.69 232.78 337.80 401.94 35.1 -162.8 163.6 62.8 +9.6 70.42 112.1 -47.3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
K ...View the full answer

Answered By

PRINCE PANDEY
I am Indian Chartered Accounting having a strong hold in the subjects of Accounting, IFRS Reporting, Indian
Taxation, Cost Accounting, Auditing. I have vast experience of teaching a student with easy way problem-solving approach.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Use the data in Appendix 2A to calculate the standard reaction enthalpy for the reaction of magnesium carbonate with hydrochloric acid: MgCO3 (s) + 2 HCI (aq) MgCl (aq) + HO(1) + CO(g)
-
Use the data in Appendix 2A to calculate the standard reaction enthalpy for the reaction of pure nitric acid with hydrazine: 4 HNO3(1) + 5 NH4 (1) 7 N(g) + 12 HO(1)
-
Assume that H and S are independent of temperature and use data in Appendix 2A to calculate G for each of the following reactions at 250. C. Over what temperature range will each reaction be...
-
Evaluate Lim- X-0 b) Evaluate Lim t-1 4 3 10x3-8x4+2x3 c) Evaluate Lim 2 2 15x4+3x3-5x 2t +21 +5t+5 t+t-71-7 -4 5m +4m +18m m 6m + 4m-6 If lim [x -f(x)] = 2, use the Rules of Limits to evaluate...
-
Costs of quality (CMA, adapted) Costen, Inc., produces cell phone equipment. Jessica Tolmy, Costens president, decided to devote more resources to the improvement of product quality after learning...
-
How far does the runner whose velocity-time graph is shown in Figure travel in 16 s? The figure's vertical scaling is set by vs = 8.0m/s. 12 16 t (s) (s/u) A
-
What are some of the principal reasons why project management has become such a popular business tool in recent years?
-
Keesha Co. borrows $200,000 cash on November 1, 2015, by signing a 90-day, 9% note with a face value of $200,000. 1. On what date does this note mature? (Assume that February of 2015 has 28 days.) 2....
-
Explain why you agree or disagree with the following statement: "Since mortgage passthrough securities issued by Ginnie Mae are guaranteed by the full faith and credit. the U.S. government, there is...
-
A fuel oil is analyzed and found to contain 85.0 wt% carbon, 12.0% elemental hydrogen (H), 1.7% sulfur, and the remainder noncombustible matter. The oil is burned with 20.0% excess air, based on...
-
Rank the following solutions in order of increasing pH: (a) 1.0 * 10 5 m NaOH(aq); (b) 0.20 m NaNO 2 (aq); (c) 0.20 m NH 3 (aq); (d) 0.20 m NaCN(aq). Justify your ranking.
-
For the exercise in this topic, base your answers on the potentials listed in Table 6M.1 or Appendix 2B, with the exception of the reduction and oxidation of water at pH = 7: Suppose that 2.69 g of a...
-
A 2005 AP-IPSOS poll found that 21% of American adults surveyed said their child was heavier than doctors recommend. The reasons given as the most important contributing factor to the childs weight...
-
Complete the "Leadership Vision Questionnaire" in Chapter 7 (p176). Reflect on your results and complete the following prompts: Share the results from your questionnaire. Be sure to include the final...
-
1. Prepare el Presupuesto Operacional hasta completar el COGS (70 puntos) La empresa ACCO 295 tiene una venta proyectada de $450,000 Cada unidad se vende $450 Su inventario inicial 300 (costo $125)...
-
Continuing Case 65. Retirement Income Forecast Jamie Lee and Ross, now 57 and still very active, have plenty of time on their hands now that the triplets are away at college. They both realized that...
-
The partnership of Frick, Wilson, and Clarke has elected to cease all operations and liquidate its business property. A balance sheet drawn up at this time shows the following account balances: Cash...
-
Harry and Sally went to a large hardware store and told the salesperson they wanted the cheapest rotating clothesline in stock, provided it would bear a heavy load of washing. The salesperson assured...
-
Compute the standard error of (1 - 2) for the following data: Sample1 Sample 2 47 6.5 8.4
-
A 20-cm-square vertical plate is heated to a temperature of 30oC and submerged in glycerin at 10oC. Calculate the heat lost from both sides of the plate.
-
Determine the structures of compounds A through F: Na,Cr,0, H2SO4, H20 [H'] A EtO soch 1) LIAI(OR)H `2) H20 xS NH3
-
Identify the reagents you would use to convert 1-bromopentane into each of the following compounds: (a) Pentanoic acid (b) Hexanoic acid (c) Pentanoyl chloride (d) Hexanamide (e) Pentanamide (f)...
-
Starting with benzene and using any other reagents of your choice, show how you would prepare each of the following compounds: a. b. c. d.
-
Describe how the following affect the valuation of PPE. a) Cash Discounts b) Deferred Payment Contracts
-
Lou Barlow, a divisional manager for Sage Company, has an opportunity to manufacture and sell one of two new products for a five - year period. His annual pay raises are determined by his division s...
-
Consider a 5 year debt with a 15% coupon rate paid semi-annually, redeemable at Php1,000 par. The bond is selling at 90%. The flotation cost is Php50 per bind. The firm's tax bracket is 30%.

Study smarter with the SolutionInn App


