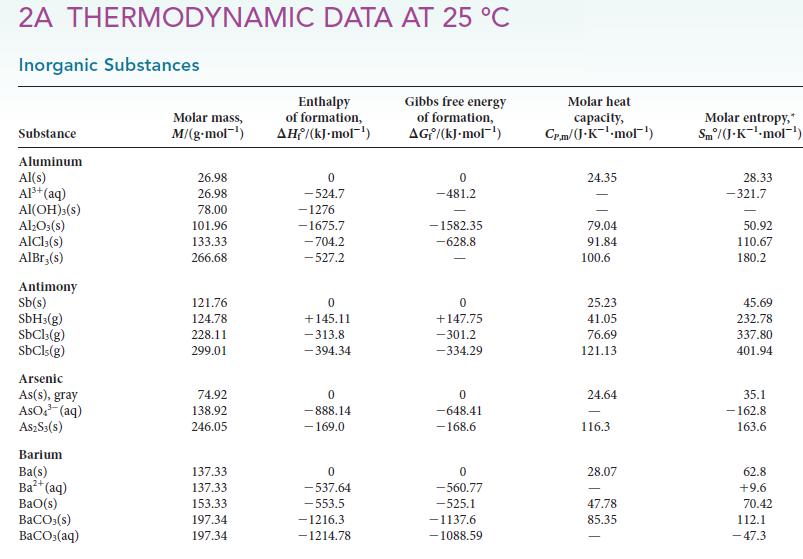
Use the data in Appendix 2A to calculate the standard reaction enthalpy for the reaction of pure
Question:
Use the data in Appendix 2A to calculate the standard reaction enthalpy for the reaction of pure nitric acid with hydrazine:![]()
Transcribed Image Text:
4 HNO3(1) + 5 N₂H4 (1) 7 N₂(g) + 12 H₂O(1)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)
298...View the full answer

Answered By

Bhartendu Goyal
Professional, Experienced, and Expert tutor who will provide speedy and to-the-point solutions. I have been teaching students for 5 years now in different subjects and it's truly been one of the most rewarding experiences of my life. I have also done one-to-one tutoring with 100+ students and help them achieve great subject knowledge. I have expertise in computer subjects like C++, C, Java, and Python programming and other computer Science related fields. Many of my student's parents message me that your lessons improved their children's grades and this is the best only thing you want as a tea...
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Use the data in Appendix 2A to calculate the standard reaction enthalpy for the reaction of magnesium carbonate with hydrochloric acid: MgCO3 (s) + 2 HCI (aq) MgCl (aq) + HO(1) + CO(g)
-
Use standard enthalpies of formation from Appendix 2A to calculate the standard reaction enthalpy for each of the following reactions: (a) The final stage in the production of nitric acid: (b) The...
-
Use data in Table 4H.1 or Appendix 2A to calculate the standard entropy change for each of the following reactions at 25C. For each reaction, interpret the sign and magnitude of the reaction entropy....
-
A general ledger trial balance at June 30, 2011, for Millar City is as follows: Millar City uses a purchases basis in accounting for supplies. Open encumbrances are considered constrained by the...
-
Data for Sanchez Manufacturing are given in BE21-1. Supporting records show that (a) The Assembly Department used $24,000 of raw materials and $30,000 of the factory labor, and (b) The Finishing...
-
Determine whether the statement is true or false. If it is true, explain why. If it is false, explain why or give an example that disproves the statement. To evaluate we can use the formula in entry...
-
5. Bec, an active partner in the Bec and Cri partnership, receives an annual bonus of 25 percent of partnership net income after deducting the bonus. For the year ended December 31, 2016, partnership...
-
The PDC Company was described earlier in this chapter. Refer to the PDC Companys projected monthly operating schedules in Table. PDCs sales are projected to be $80,000 in September 2011. A. Prepare...
-
The control of Mercury Shoes Inc. instructs you to prepare a monthly cash budget for the next three months. You are presented with the following buget information: Jund July August $160,000 $185,000...
-
Calculate the standard entropy of vaporization of water at 85C, given that its standard entropy of vaporization at 100.C is 109.0 J K 1 mol 1 and the molar heat capacities at constant pressure of...
-
Use the following information to construct a heating curve for bromine from 27.2C to 70.0C. The molar heat capacity of liquid bromine is 75.69 J K 1 mol 1 and that of bromine vapor is 36.02 J K 1 ...
-
Write the structural formula and name the organic product expected from the acid-catalyzed condensation reaction of CH 3 OH.
-
15.5 please help will give like if answers r correct Exercise 15-8 (Static) Sales-type lease with selling profit; lessor; calculate lease payments [LO15-3] Manufacturers Southern leased high-tech...
-
When my son was young, he had 8 different plastic dinosaurs to arrange. How many ways could he arrange his 8 dinos? He had favorite dinos, so placing them in proper order was very important. How many...
-
Process P1 init (mutEx); num = 0; loop1 = 0; while (loop1 < 3) wait (mutEx); num num + 1; signal (mutEX); loop1 loop1 + 1; Process P2 loop2 = 0; while (loop2 < 2) wait (mutEx); num num + 10;...
-
PROBLEM 3-5B Following is the chart of accounts of Smith Financial Services: Assets 111 Cash 113 Accounts Receivable 115 Supplies 117 Prepaid Insurance 124 Office Furniture Liabilities 221 Accounts...
-
4. Identify a service you could refer Casey to and write a referral for her (up to 300 words).
-
Acute murine leukemia virus (AMLV) causes leukemia in mice. This virus is easily passed from mother to offspring through the mother's milk. (Even though newborn offspring acquire the virus, they may...
-
At Glass Company, materials are added at the beginning of the process and conversion costs are added uniformly. Work in process, beginning: Number of units Transferred - in costs Direct materials...
-
Define and give an example of each of the following. a. Addition polymer b. Condensation polymer c. Copolymer d. Homopolymer e. Polyester f. Polyamide
-
What is polystyrene? The following processes result in a stronger polystyrene polymer. Explain why in each case. a. Addition of catalyst to form syndioracric polystyrene b. Addition of 1,3-butadiene...
-
What monomer(s) must be used to produce the following polymers? a. b. c. d. e. f. Classify these polymers as condensation or addition polymers. Which are copolymers? CH-CH2-CH-CH-CH-CH O-CH2-CH2-C-o...
-
please help Problem 13-7 (Algo) Prepare a Statement of Cash Flows [LO13-1, LO13-2] [The following information applies to the questions displayed below.] Comparative financial statements for Weaver...
-
A firm has 1000 shareholders, each of whom own $59 in shares. The firm uses $28000 to repurchase shares. What percentage of the firm did each of the remaining shareholders own before the repurchase,...
-
Vancouver Bank agrees to lend $ 180,000 to Surrey Corp. on November 1, 2020 and the company signs a six-month, 6% note maturing on May 1, 2021. Surrey Corp. follows IFRS and has a December 31 fiscal...

Study smarter with the SolutionInn App


