Assume that H and S are independent of temperature and use data in Appendix 2A to calculate
Question:
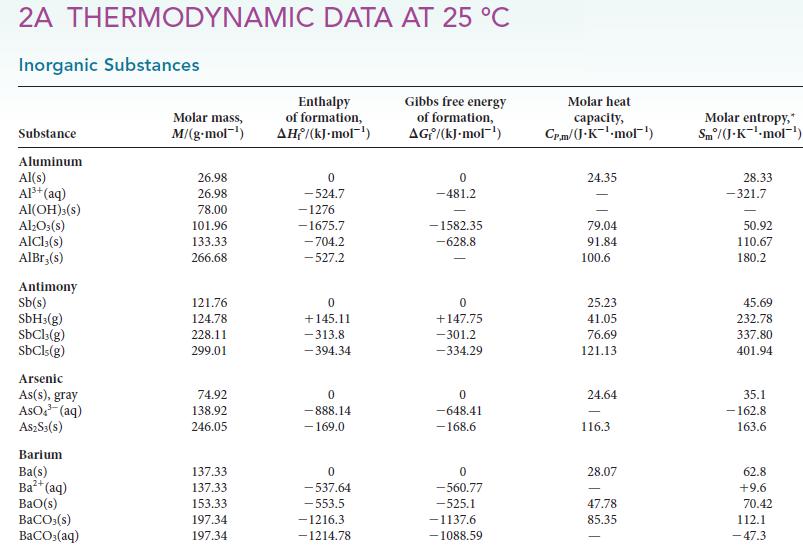
Assume that ΔH° and ΔS° are independent of temperature and use data in Appendix 2A to calculate ΔG° for each of the following reactions at 250. °C. Over what temperature range will each reaction be spontaneous under standard conditions?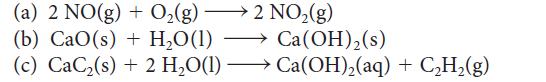
Transcribed Image Text:
(a) 2 NO(g) + O(g). 2 NO(g) (b) CaO(s) + HO(1) (c) CaC(s) + 2 HO(1) Ca(OH) (s) Ca(OH)(aq) + CH(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
a AHr ASF 23318 kJ mol29025kJ mol 11414kJ mol1 224006J K1 mol1 221076J K mol 20514J Kmol1 14654 J K1...View the full answer

Answered By

Pushpinder Singh
Currently, I am PhD scholar with Indian Statistical problem, working in applied statistics and real life data problems. I have done several projects in Statistics especially Time Series data analysis, Regression Techniques.
I am Master in Statistics from Indian Institute of Technology, Kanpur.
I have been teaching students for various University entrance exams and passing grades in Graduation and Post-Graduation.I have expertise in solving problems in Statistics for more than 2 years now.I am a subject expert in Statistics with Assignmentpedia.com.
4.40+
3+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Assume that H and S are independent of temperature and use data in Appendix 2A to calculate G for each of the following reactions at 80.C. Over what temperature range will each reaction be...
-
Use the standard Gibbs free energies of formation in Appendix 2A to calculate G for each of the following reactions at 25 C. Comment on the spontaneity of each reaction under standard conditions at...
-
Use the standard Gibbs free energies of formation in Appendix 2A to calculate G for each of the following reactions at 25 C. Comment on the spontaneity of each reaction under standard conditions at...
-
B-bol King Co. started a new promotional program. For every 10 box tops returned, customers receive a basketball. The entity estimated that only 60% of the box tops reaching the market will be...
-
Ohio Glass Company manufactures three types of safety plate glass: large, medium, and small. All three products have high demand. Thus, Ohio Glass is able to sell all the safety glass that it can...
-
Write a program that animates the AVL tree insert, delete, and search methods, as shown in Figure 26.2. D AVL Tree Animation by Y C O liveexample.pearsoncmg.com/dsanimation/AVLTreeeBook.html Q * O...
-
Why would the members of a NOT work independently if they were members of a designated team? What does independently mean in this context? LO6
-
Peach Corporation (a calendar year company) recorded the following transactions. Taxable income ...................... $5,000,000 Regular tax depreciation on realty in excess of ADS (placed in...
-
Wildhorse Corporation produces industrial robots for high-precision manufacturing. The following information is given for Wildhorse Corporation: Per Unit Total $400 300 80 Direct materials Direct...
-
A technician carries out the reaction 2 SO 2 (g) + O 2 (g) 2 SO 3 (g) at 25C and 1.00 atm in a cylinder fitted with a piston and maintained at constant pressure. Initially, 0.030 mol SO 2 and 0.030...
-
Use only your knowledge of intermolecular forces to rank the following compounds in order of increasing enthalpy of vaporization of their liquid state: CH 4 , H 2 O, N 2 , NaCl, C 6 H 6 , and H 2 ....
-
You are installing a new spark plug in your car, and the manual specifies that it be tightened to a torque that has a magnitude of 45 N m. Using the data in the drawing, determine the magnitude F of...
-
Explain the role of EHR healthcare technology in the delivery of care
-
In the movie, Money Ball what was the change that the Oakland A's was going through under the leadership of Billy Beane? 2.: In leading the change that you described in Q1, what was the...
-
Studies of the grapevine network within organizations have shown that the rumours and gossip on the grapevine are almost always accurate, and that a prudent manager is wise to act on that...
-
What is Program Evaluation? Describe What is need assessment? Describe? What is a program logic model? Describe and analyze. What is one example? (including input, output, short term outcomes and...
-
What are the primary jobs that must be performed at Spotify? Using the job characteristics theory as a frame-work, assess these jobs in terms of their motivating potential. 2. How does the concept of...
-
Write the formulas for each of the following ions and compounds: (a) Tetrahydroxozincate(II), (b) Pentaaquachlorochromium(III) chloride, (c) tetrabromocuprate( II), (d)...
-
(a) Prove that form an orthonormal basis for R3 for the usual dot product. (b) Find the coordinates of v = (1, 1, 1)T relative to this basis. (c) Verify formula (5.5) in this particular case. 48-65...
-
Consider \(100.0 \mathrm{~mL}\) of a solution of \(0.200 \mathrm{M} \mathrm{Na}_{2} \mathrm{~A}\), where \(\mathrm{A}^{2-}\) is a base with corresponding acids \(\mathrm{H}_{2} \mathrm{~A}\)...
-
The titration of \(\mathrm{Na}_{2} \mathrm{CO}_{3}\) with \(\mathrm{HCl}\) has the following qualitative profile: a. Identify the major species in solution as points A-F. b. For the titration of...
-
A student was given a \(0.10 \mathrm{M}\) solution of an unknown diprotic acid \(\mathrm{H}_{2} \mathrm{~A}\) and asked to determine the \(K_{\mathrm{a}_{1}}\) and \(K_{\mathrm{a}_{2}}\) values for...
-
You have just been hired as a new management trainee by Earrings Unlimited, a distributor of earrings to various retail outlets located in shopping malls across the country. In the past, the company...
-
Brief Exercise 10-6 Flint Inc. purchased land, building, and equipment from Laguna Corporation for a cash payment of $327,600. The estimated fair values of the assets are land $62,400, building...
-
"faithful respresentation" is the overriding principle that should be followed in ones prepaparation of IFRS-based financial statement. what is it? explain it fully quoting IAS. how this this...

Study smarter with the SolutionInn App


