Assume that H and S are independent of temperature and use data in Appendix 2A to calculate
Question:
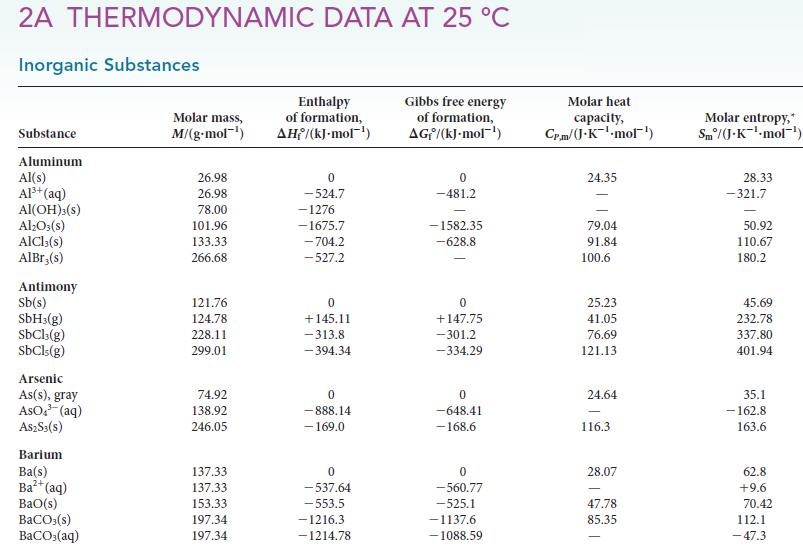
Assume that ΔH° and ΔS° are independent of temperature and use data in Appendix 2A to calculate ΔG° for each of the following reactions at 80.°C. Over what temperature range will each reaction be spontaneous under standard conditions?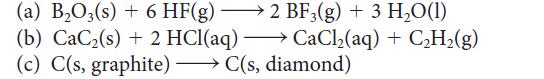
Transcribed Image Text:
(a) B₂O3(s) + 6 HF(g) → 2 BF3(g) + 3 H₂O(1) (b) CaC₂ (s) + 2 HCl(aq) →→→ CaCl₂(aq) + C₂H₂(g) diamond) - (c) C(s, graphite)C(s,
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (6 reviews)
a AG 9842 kJmol spontaneous ...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Assume that H and S are independent of temperature and use data in Appendix 2A to calculate G for each of the following reactions at 250. C. Over what temperature range will each reaction be...
-
Use the standard Gibbs free energies of formation in Appendix 2A to calculate G for each of the following reactions at 25 C. Comment on the spontaneity of each reaction under standard conditions at...
-
Use the standard Gibbs free energies of formation in Appendix 2A to calculate G for each of the following reactions at 25 C. Comment on the spontaneity of each reaction under standard conditions at...
-
Compute the least-squares regression line for predicting power (y) from wind speed (x).
-
Toyota Motor Corporation uses target costing. Assume that Toyota marketing personnel estimate that the competitive selling price for the Camry in the upcoming model year will need to be $22,000....
-
Design a class named Date that meets the following requirements: Three data fields year, month, and day for representing a date A constructor that constructs a date with the specified year, month,...
-
How should a PM decide which problems (or potential problems) deserve being reported to management and which are not worth the trouble when attempting to never surprise the boss? LO6
-
Heller Manufacturing has two production facilities that manufacture baseball gloves. Production costs at the two facilities differ because of varying labor rates, local property taxes, type of...
-
NUMERICAL EXERCISE If 1 OMR is equal to 2.54 USD, then find the value of 3000 OMR value in USD and 200USD in OMR. If 20000 SAR is equal to 5500 USD and 4500 Pound find the value 5000 SAR in USD and...
-
Which of the following compounds become less stable with respect to their elements as the temperature is raised: (a) C 3 H 6 (g), cyclopropane; (b) CaO(s); (c) N 2 O(g); (d) HN 3 (g)?
-
Hydrochloric acid oxidizes zinc metal in a reaction that produces hydrogen gas and chloride ions. A piece of zinc metal of mass 8.5 g is dropped into an apparatus containing 800.0 mL of 0.500 m...
-
By the time you are in college, you are in charge of at least some of your own finances. How well you manage your personal budget may indicate how well you will manage your companys budget on the...
-
a) Discuss whether bike paths can be considered a public good. Now consider a hypothetical town. Suppose that there are three equal-size groups in the economy with the following demand curves: Group...
-
Event services and management can be a lucrative revenue generator. What are the two most important factors in developing a successful event service and management business, whether it is independent...
-
Show how the buying process occurs in the consumer. Review some of the steps in the buying process, stories like: felt need pre-purchase activity purchase decision Post-purchase feelings Explain and...
-
How did Henry Ford set the stage for some of the same problems we still face today in employee relations, especially in manufacturing? 2) If you were a human resources manager, how would you address...
-
What does a DMO risk by not having a positioning theme? Critique the potential of your destination's slogan to effectively differentiate against rivals. you have been asked by a television network to...
-
Give the oxidation numbers of the metals in the following species: (a) Na2MoO4, (b) MgWO4, (c) Fe(CO)5.
-
For the vector whose polar components are (Vr = 1, Vθ = 0), compute in polars all components of the second covariant derivative Vα;μ;ν. To find...
-
Consider a weak acid HA with a \(K_{a}\) value of \(1.6 \times 10^{-7}\). Calculate the \(\mathrm{pH}\) of a solution that is \(5.0 \times\) \(10^{-7} \mathrm{M} \mathrm{HA}\) and \(5.0 \times...
-
In Section 8.3 an equation was derived for the exact treatment of HA/NaA-type buffers. What would be the expression for \(\mathrm{B} / \mathrm{BHCl}\)-type buffers stated in terms of...
-
Which of the following mixtures would result in a buffered solution when \(1.0 \mathrm{~L}\) of each of the two solutions are mixed? a. \(0.1 \mathrm{M} \mathrm{KOH}\) and \(0.1 \mathrm{M}...
-
Determine the simple interest earned on $10,000 after 10 years if the APR is 15%
-
give me an example of 10 transactions from daily routine that we buy and put for me Liabilities + Owners' Equity + Revenues - Expenses
-
What is the Macaulay duration of a bond with a coupon of 6.6 percent, seven years to maturity, and a current price of $1,069.40? What is the modified duration? (Do not round intermediate...

Study smarter with the SolutionInn App


