For the oxidationreduction reaction the appropriate half-reactions are Balance the redox reaction and calculate and K
Question:
For the oxidation–reduction reaction
![]()
the appropriate half-reactions are
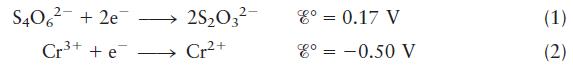
Balance the redox reaction and calculate ξ° and K (at 25°C).
Transcribed Image Text:
S406 (aq) + Cr+ (aq) 3+ Cr+ (aq) + S03 (aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (4 reviews)
To obtain the balanced reaction we must reverse rea...View the full answer

Answered By

BETHUEL RUTTO
Hi! I am a Journalism and Mass Communication graduate; I have written many academic essays, including argumentative essays, research papers, and literary analysis. I have also proofread and written reviews, summaries and analyses on already finished works. I am eager to continue writing!
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Explain how the following Arduino codes affect the motion of the motor. Which direction is the motor moving? Why the map commands are required? (6%) duty1 = 40; duty2 = 60; dutyl-map...
-
Figure above shows a simply supported beam being loaded a shown. The cross-section of the beam is also shown above. It is doubly symmetrical. Determine the vertical reactions at A and D in kN. Draw...
-
Write two suitable solutions to overcome each of the following problems: 1. Poor dispersion of reinforcement during the production of PMC's using an internal mixer. 2. Poor impregnation of...
-
Describe what happens if you apply binary search to an unordered array. Why shouldn't you check whether the array is sorted before each call to binary search? Could you check that the elements binary...
-
A solid cube of uniform density and sides of b is in equilibrium on top of a cylinder of radius R (Figure 2-C). The planes of four sides of the cube are parallel to the axis of the cylinder. The...
-
Modify and solve the capital budgeting model of Example 9.1-1 to account for the following additional restrictions: (a) Project 5 must be selected if either project 1 or project 3 is selected. (b)...
-
1 2 Compare internationalisation and globalisation. Give a specific example of a company of each type about which you have obtained some information.
-
Joe Schmaltz has carried on a retail business for about 20 years. He intends to transfer the business assets and liabilities to a corporation, Schmaltz Enterprises Ltd. (SEL), in which he will own...
-
TB MC Qu. 5-125 (Algo) Mcdale Incorporated produces and sells two... Mcdale Incorporated produces and sells two products. Data concerning those products for the most recent month appear below:...
-
Determine the direction of electron flow, designate the anode and cathode, and calculate the potential at 25C for the cell represented in Fig. 11.12. Fe- 0.01 M Fe+ FIGURE 11.12 Porous disk -Fe 0.1 M...
-
Describe the cell based on the following half-reactions: where VO+ + 2H+ + e Zn+ +2e= VO+ + HO 2+ Zn 8 = 1.00 V 8 = -0.76 V (1) (2)
-
Suppose you and your best friend decide to start a house-painting service. Draft a production plan for your business, including the following decisions: (a) Make, buy, or lease; (b) Suppliers; and...
-
Why the sudden increase in income before taxes in 2021? 8. Why were the operating assets the highest in 2019? 9. Why are the short-term loans the highest in 2020? 10. Why are the other long-term...
-
Mercy wants to make sure that she will be able to provide for her daughter's college and plans to open a savings account with a bank that is ready to pay interest as shown below per year compounded...
-
Question 1. For a firm that uses portfolio management, please give a real or hypothetical example of how the CEO's personal bases for power help organizational performance. Question 2. Give a real...
-
Make a schedule that you would use that effectively illustrates working with paraprofessionals that includes collaboration time. Use the examples provided in the following resources to guide your...
-
How does the integration of technology and automation influence employee motivation and job satisfaction within modern organizational contexts ?
-
Reconsider Prob. 10-31. Using EES (or other) software, solve this problem by the diagram window data entry feature of EES. Include the effects of the turbine and pump efficiencies and also show the...
-
What are the 5 Cs of marketing channel structure?
-
Like sulfuric acid, a certain diprotic acid, H 2 A, is a strong acid in its first deprotonation and a weak acid in its second deprotonation. A solution that is 0.015 m H 2 A(aq) has a pH of 1.72....
-
Arrange the following species in order of increasing strength as oxidizing agents for species in aqueous solution: (a) Co 2+ , Cl 2 , Ce 4+ , In 3+ ; (b) NO 3 , ClO4 , HBrO, Cr 2 O7 2 , all in...
-
Below are molecular models of two oxoacids. Write the name of each acid and then draw the model of its conjugate base. (Red = O, white = H, green = Cl, and blue = N.) (a) (b)
-
Aecerty 1067687 was completed with the folowing charaderistick Murulectere sec00 5xs:99 s35ida sputed
-
Assume todays settlement price on a CME EUR futures contract is $1.3180 per euro. You have a long position in one contract. EUR125,000 is the contract size of one EUR contract. Your performance bond...
-
Q2. Company ABC bought an equipment for $20,000 in 2015, with useful life of 5 years $5,000 residual value amortized using straight-line method. Prepare a table to illustrate the differences...

Study smarter with the SolutionInn App


