Describe the cell based on the following half-reactions: where VO+ + 2H+ + e Zn+ +2e= VO+
Question:
Describe the cell based on the following half-reactions:
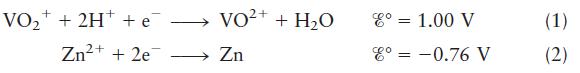
where
![T = 25C [VO+] = 2.0 M [H] = 0.50 M [VO+] = 1.0 x 10- M [Zn+] = 1.0 10- M](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1706/0/8/9/95565b0dde3d628f1706089955983.jpg)
Transcribed Image Text:
VO+ + 2H+ + e Zn+ +2e= VO+ + HO 2+ Zn 8 = 1.00 V 8 = -0.76 V (1) (2)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (2 reviews)
The balanced cell reaction is obtained by reversing reaction 2 and multiplyi...View the full answer

Answered By

Sarfraz gull
have strong entrepreneurial and analytical skills which ensure quality tutoring and mentoring in your international business and management disciplines. Over last 3 years, I have expertise in the areas of Financial Planning, Business Management, Accounting, Finance, Corporate Finance, International Business, Human Resource Management, Entrepreneurship, Marketing, E-commerce, Social Media Marketing, and Supply Chain Management.
Over the years, I have been working as a business tutor and mentor for more than 3 years. Apart from tutoring online I have rich experience of working in multinational. I have worked on business management to project management.
5.00+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
KYC's stock price can go up by 15 percent every year, or down by 10 percent. Both outcomes are equally likely. The risk free rate is 5 percent, and the current stock price of KYC is 100. (a) Price a...
-
Consider the standard galvanic cell based on the following half reactions Cu2+ + 2e- Cu Ag+ + e- Ag The electrodes in this cell are Ag(s) and Cu(s). Does the cell potential increase, decrease, or...
-
The Stand-Alone Project requires you to perform an in-depth strategic audit of American Airlines, Inc. These past several years have been troubling times for the airline industry. Jet fuel prices...
-
Modify Index to make a program IndexByKeyword that takes a file name from the command line and makes an index from standard input using only the keywords in that file. Note: Using the same file for...
-
A train moves along the tracks at a constant speed v. A woman on the train throws a ball of mass m straight ahead with a speed v with respect to herself. (a) What is the kinetic energy gain of the...
-
In Problem 10, suppose that the nature of the melodies dictates that songs 3 and 4 cannot be recorded on the same side. Formulate the problem as an ILP. Would it be possible to use a 25-minute tape...
-
1 1 What factors are stimulating the growth in world trade?
-
Shirtstop makes T-shirts with logos and sells them in its chain of retail stores. It contracts with two different plantsone in Puerto Rico and one in The Bahamas. The shirts from the plant in Puerto...
-
Sheffield Company reports the following for the month of June. Units Unit Cost Total Cost June 1 Inventory 200 $7 $ 1,400 12 Purchase 400 8 3,200 23 Purchase 330 9 2,970 30 Inventory 100 (a) Compute...
-
For the oxidationreduction reaction the appropriate half-reactions are Balance the redox reaction and calculate and K (at 25C). S406 (aq) + Cr+ (aq) 3+ Cr+ (aq) + S03 (aq)
-
For the cell reaction predict whether cell will be larger or smaller than cell for the following cases. 2Al(s) + 3Mn+ (aq) 2A1+ (aq) + 3Mn(s) Ecell = 0.48 V
-
Find an equation of the hyperbola with foci (0, 5) and vertices (0, 2).
-
BREAD Products' pretax income for 2019 is * (1 Point) BREAD Products has no Work in Process or Finished Goods inventories at the close of business on December 31, 2018. The balances of BREAD's...
-
Convert the following line of code into assembly language. A (A B)+(BA) Where A and B are both 8-bit variables Activate Windows
-
14. Create a one variable Data Table from what you just copied and pasted giving the total sales for each department, and the Largest Sale from each department. Start your Criteria range in cell A1....
-
E4.1 (LO 1), C The following independent situations require professional judgment for determining when to recognize revenue from the transactions. a. Southwest Airlines sells you an advance-purchase...
-
Spring Flings Company, a fashion retailer that specializes in colorful graphic tees, prepares a master budget on a quarterly basis. The company has assembled the following data to assist in preparing...
-
A steam power plant operates on the ideal reheat Rankine cycle. Steam enters the high pressure turbine at 6 MPa and 400C and leaves at 2 MPa. Steam is then reheated at constant pressure to 400C...
-
What is your opinion of advertising awards, such as the Cannes Lions, that are based solely on creativity? If you were a marketer looking for an agency, would you take these creative awards into...
-
Many reactions that take place in water have analogous reactions in liquid ammonia (normal boiling point, 33C). (a)Write the chemical equation for the autoprotolysis of NH 3 . (b)Write the formulas...
-
The pH of several solutions was measured in a hospital laboratory; convert each of the following pH values into the molar concentration of H 3 O + ions: (a) 4.8 (the pH of a urine sample); (b) 0.7...
-
Use data from Tables 6C.1 and 6C.2 to place the following acids in order of increasing strength: HCOOH, (CH 3 ) 3 NH + , N 2 H 5 + , HF. TABLE 6C.1 Acidity Constants at 25 C* K 3.0 X 10-1 2.0 X 10-...
-
Question 7 of 7 0/14 W PIERDERY Current Attempt in Progress Your answer is incorrect Buffalo Corporation adopted the dollar value LIFO retail inventory method on January 1, 2019. At that time the...
-
Cost of debt with fees . Kenny Enterprises will issue a bond with a par value of $1,000, a maturity of twenty years, and a coupon rate of 9.9% with semiannual payments, and will use an investment...
-
Assume that an investment of $100,000 is expected to grow during the next year by 8% with SD 20%, and that the return is normally distributed. Whats the 5% VaR for the investment? A. $24,898 B....

Study smarter with the SolutionInn App


