Given the following data, calculate the value for the overall formation constant for (mathrm{Mn}left(mathrm{C}_{2} mathrm{O}_{4}ight)_{2}{ }^{2-}) :
Question:
Given the following data,
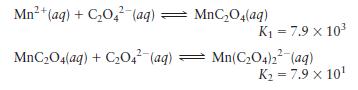
calculate the value for the overall formation constant for \(\mathrm{Mn}\left(\mathrm{C}_{2} \mathrm{O}_{4}ight)_{2}{ }^{2-}\) :
\[K=\frac{\left[\mathrm{Mn}\left(\mathrm{C}_{2} \mathrm{O}_{4}ight)_{2}^{2-}ight]}{\left[\mathrm{Mn}^{2+}ight]\left[\mathrm{C}_{2} \mathrm{O}_{4}{ }^{2-}ight]^{2}}\]
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





