Given the following data: calculate DH for the reaction CH(g) + O(g) C(s) + O(g) H(g) +
Question:
Given the following data:
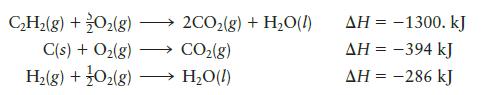
calculate DH for the reaction
![]()
Transcribed Image Text:
C₂H₂(g) + O₂(g) C(s) + O₂(g) H₂(g) + O₂(g) - →2CO₂(g) + H₂O(l) CO₂(g) → H₂O(l) AH = -1300. kJ AH = -394 kJ AH = -286 kJ
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (5 reviews)
Given the following data Eq1 C2H2 g 32O2g CO2g H2O l H...View the full answer

Answered By

Godswill Okorie
M.Sc chemistry specialization in organic chemistry, B.ed .I am having industry experience of seven years working with Ranbaxy nd Shimadzu analytical India by working as an application chemistry.I am having good practical experience on chromatography techniques,which later helped me in my teaching.I worked as PGT chemistry teacher with KV and APS.
As a teacher I was able to achieve good results with my students.I used to take 11th and 12th chemistry and science to classes 7th ,8th and ninth. While teaching I used to guide students for various carrier opportunities.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Given the following data from three in de pen dent samples, use the 0.025 level in determining whether the population means could be the same. (Use data file XR12092.) 3-48377 11212 701536 21121 2...
-
Given the following data on inputs and outputs at a work center, determine the cumulative deviation and the backlog for each time period. The beginning backlog is7. PERIOD Input Planned2 Actua2 200...
-
Given the following data from group 1 and group 2: a. Combine the two datasets in ranked order. Compute the sum of the ranks for group 1, R1. Compute the sum of the ranks for group 2, R2. b. Compute...
-
Find the magnitude and direction of the electric field strength at the point P due to the point charges at A and B as shown in the Figure 1. (k = 9 x 10 Nm/C) +2 C 10 cm A 10 cm Figure 1 B -8 C (5...
-
Suppose that a 2 test of independence at the level of significance 0.005 is to be applied to the elements of a 2 2 contingency table containing 2n observations, and that for some (0, 1) the data...
-
Finns Seafood Restaurant has been approached by New England Investments, which wants to hold an employee recognition dinner next month. Lillian Sumner, a manager of the restaurant, agreed to a charge...
-
What is the traditional lodging reservation system? How do you think the Internet will change the reservation system?
-
Given a sample size of 36, how large does the population standard deviation have to be in order for the standard error to be (a) 1 (b) 2 (c) 5 (d) 100
-
DO NOT USE EXCEL TO SOLVE THE PROBLEM. I CANNOT USE EXCEL ON AN EXAM. $150000 was borrowed at 8% per year. Annual end of year payments of $14700 will be made. When this loan is paid off, what will be...
-
a. Using the data in the income statement and the balance sheet that follow, compute the companys average collection period (ACP) in days. Use a 365-day year when calculating sales per day. b....
-
Given the following data: calculate DH for the reaction 2CIF(g) + O(g) 2CIF3(g) + 202(8) 2F(g) + O(g) ClO(g) + FO(g) ClO(g) + 3FO(g) 2FO(g) = 167.4 kJ = 341.4 kJ AH = -43.4 kJ
-
Given the following data: calculate DH for the reaction On the basis of enthalpy change, is this a useful reaction for the synthesis of ammonia? (g) + N (g) = 92 kJ (g) = 484 kJ
-
Assume that Horicon Corp acquired 25% of the common stock of Sheboygan Corp. on January 1, 2014, for $300,000. During 2014 Sheboygan Corp. reported net income of $160,000 and paid total dividends of...
-
Question: 9. Purchases and sales during a recent period for Bottineau Inc. were Purchases During the Period Sales During the Period 1st purchase 1,500 units x $ 4 1st sale 700 units x $13 2nd...
-
# The following is a partial relative frequency distribution of consumer preferences for four products-A, B, C, and D. Required: Determine the relative frequency for Product B: Relative Frequency...
-
Domino Company's operating percentages were as follows: Revenues 100% Cost of goods sold Variable 50% Fixed 10% 60% Gross profit 40% Other operating expenses Variable 20% Fixed 15% 35% Operating...
-
Marcus Stewart, the production manager at Galvin Company, purchased a cutting machine for the company last year. Six months after the purchase of the cutting machine, Stewart learned about a new...
-
The TechTeach Company produces and sells 7,000 modular computer desks per year at a selling price of $750 each. Its current production equipment, purchased for $1,950,000 and with a 5-year useful...
-
A light bulb emits a spherical wave. If the intensity of the emitted light is 1.0 W/m 2 at a distance of 2.5 m from the bulb, what is the intensity at a distance of 4.0 m?
-
In a paragraph of approximately 150-200 words, analyze a film or TV/Streaming Show poster of your choosing by focusing on the ways in which representations in the poster are gendered. Include an...
-
Explain the photoelectric effect.
-
An atom moving at its root mean square velocity at 100oC has a wavelength of 2.31 10-11 m. Which atom is it?
-
The ground state ionization energy for the one electron ion Xm+ is 4.72 104 kJ/ mol. Identify X and m.
-
Question 3 (24 marks) Wonderful Technology Company Limited sells computers and accessories. Data of the store's operations are as follow: Sales are budgeted at $400,000 for December 2019, $420,000...
-
Kratz Manufacturing Company uses an activity-based costing system. It has the following manufacturing activity areas, related cost drivers and cost allocation rates: Activity Cost Driver Cost...
-
You are a Partner with Fix-It Consultants and have been engaged in an advisory capacity with a software company, called MoveFast. The company is seeing a sharp decline in revenue, with the primary...

Study smarter with the SolutionInn App


