Identify the reactions with K > 1 in the following list and, for each such reaction, identify
Question:
Identify the reactions with K > 1 in the following list and, for each such reaction, identify the oxidizing agent and calculate the standard cell potential.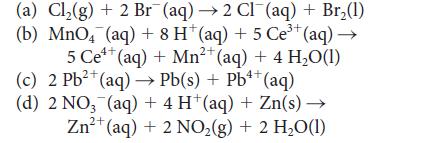
Transcribed Image Text:
(a) Cl₂(g) + 2 Br (aq) →2 Cl¯(aq) + Br₂(1) 3+ (b) MnO4 (aq) + 8 H* (aq) + 5 Ce³+ (aq) →→ 4+ 2+ 5 Ce¹+ (aq) + Mn²+ (aq) + 4 H₂O(1) 2+ 4+ (c) 2 Pb²+ (aq)→→ Pb(s) + Pb¹+ (aq) (d) 2 NO₂ (aq) + 4 H (aq) + Zn(s) → 2+ Zn²+ (aq) + 2 NO₂ (g) + 2 H₂O(1)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (6 reviews)
a Clg 027 V ...View the full answer

Answered By

Geoffrey Isaboke
I am an industrious tutor with a 5-yr experience in professional academic writing. I have passion for History and Music and I have good knowledge in Economics
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Identify the reactions with K > 1 among the following reactions and, for each such reaction, write balanced reduction and oxidation half-reactions. For those reactions, show that K > 1 by calculating...
-
Identify six (6) key areas of diversity from the following list and for each selected, give a brief description of what it means: cultural, disability, gender identity, generational, sexual identity,...
-
stock options are no longer as valuable and employees in startups are losing out to founders and early investors for all of these reasons except: there is higher employee turnover, less of a delay in...
-
A machine requires three hours to make a unit of Product A and nine hours to make a unit of Product B. Last month the machine operated for 957 hours, producing a total of 145 units. How many units of...
-
Analysis of growth, price-recovery, and productivity components (continuation of 13-23) Suppose that during 2009, the market for Merediths special-purpose machines grew by 3%. All increases in market...
-
The position vector for a proton is initially r = 5.0i 6.0j + 2.0k and then later is r = 2.0i + 6.0i + 2.0k, all in meters. (a) What is the proton's displacement vector, and (b) To what plane is...
-
Which of the four methods for handling missing data would tend to lead to an underestimate of the spread (e.g., SD) of the variable? What are some benefits to this method?
-
Access the financial statements and related disclosure notes of Google Inc. from its website at investor.google.com. In Google's balance sheet, deferred income taxes in 2008 are reported as both an...
-
Land is measured in the USA in Acres. All of the following figures represent approximately ONE ACRE of Land EXCEPTING: a. 5,280 Sq feet b. 4,850 sq yds c. 43,560 sq feet d. 4,407 Sq meter
-
Sage Hill Company specializes in manufacturing a unique model of bicycle helmet. The model is well accepted by consumers, and the company has enough orders to keep the factory production at 10,000...
-
Suppose that 25.0 mL of 0.10 m CH 3 COOH(aq) is titrated with 0.10 m NaOH(aq). (a)What is the initial pH of the 0.10 m CH 3 COOH(aq) solution? (b)What is the pH after the addition of 10.0 mL of 0.10...
-
Draw the Lewis structure or symbol for each of the following species and identify each one as a Lewis acid or Lewis base: (a) NH3; (b) BF3; (c) Ag; (d) F; (e) H.
-
One of the ratios used to indicate long-term debt-paying ability compares total liabilities to total assets. What is the intent of this ratio? How precise is this ratio in achieving its intent?
-
If f ( x ) = ( 1 3 - In ( x ) ) ^ 8 , determine f ' ( 1 ) .
-
1. ThestocksAandBhavethefollowingdistributionsofreturns. A B Probability State1 3 4 0.2 State2 5 2 0.3 State3 4 8 0.2 State4 6 5 0.1 State5 6 1 0.2 2....
-
Define nested designs. Explain why the nested designs are important.
-
3 x y 3 + x y = l n ( x ) solve for d y d x
-
Let ln ( xy ) + y ^ 8 = x ^ 7 + 2 . Find dy / dx .
-
Researchers measured the bone mineral density of the spines of 94 women who had taken the drug CEE. (See Example 6.3.4, which dealt with hip bone mineral density.) The mean was 1.016 g/cm2 and the...
-
Define the essential properties of the following types of operating systems: a. Batch b. Interactive c. Time sharing d. Real time e. Network f. Parallel g. Distributed h. Clustered i. Handheld
-
In Chapter 10, we will see that an acetylide ion (formed by treatment of acetylene with a strong base) can serve as a nucleophile in an S N 2 reaction: This reaction provides a useful method for...
-
Predict the product(s) obtained when each of the following compounds is treated with chloromethane and aluminum trichloride. Some of the compounds might be unreactive. For those that are reactive,...
-
Predict the major product obtained when each of the following compounds is treated with bromine in the presence of iron tribromide. (a) Bromobenzene (b) Nitrobenzene (c) ortho-Xylene (d)...
-
Mass LLp developed software that helps farmers to plow their fiels in a mannyue sthat precvents erosion and maimizes the effoctiveness of irrigation. Suny dale paid a licesnsing fee of $23000 for a...
-
Average Rate of Return The following data are accumulated by Lone Peak Inc. in evaluating two competing capital investment proposals: 3D Printer Truck Amount of investment $40,000 $50,000 Useful life...
-
4. (10 points) Valuation using Income Approach An appraiser appraises a food court and lounge and provides the following assessment: o O The building consists of 2 floors with the following (6)...

Study smarter with the SolutionInn App


