In theory, most metals should easily corrode in the air. Why? A group of metals called noble
Question:
In theory, most metals should easily corrode in the air. Why? A group of metals called noble metals are relatively difficult to corrode in air. Some noble metals include gold, platinum, and silver. Reference Table 11.1 to come up with a possible reason why the noble metals are relatively difficult to corrode.
Table 11.1
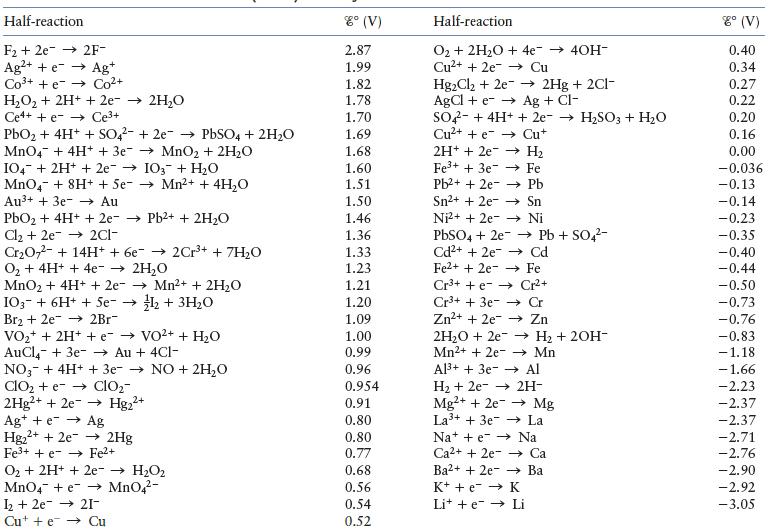
Transcribed Image Text:
Half-reaction F₂ +2e2F- Ag²+ + e- → Ag+ Co³+ + e Co²+ H₂O₂ + 2H+ + 2e → 2H₂O Ce4+ + e Ce³+ PbO₂ + 4H+ + SO4² +2e → PbSO4 + 2H₂O MnO4 + 4H+ + 3e → MnO₂ + 2H₂O IO4 + 2H+ + 2e → IO3 + H₂O MnO4 + 8H+ + Se-→ Mn²+ + 4H₂O Au³+ + 3e →→ Au PbO₂ + 4H+ + 2e-→ Pb2+ + 2H₂O Cl₂ +2e2C1- Cr₂O72- + 14H+ + 6e → 2Cr³+ + 7H₂O O₂ + 4H+ + 4e → 2H₂O MnO₂ + 4H+ + 2e- → Mn²+ + 2H₂O IO3 + 6H+ + 5e- → 1₂2 + 3H₂O Br2 + 2e 2Br- VO₂+ + 2H+ + e- → VO²+ + H₂O AuCl + 3e- → Au + 4C1- NO + 2H₂O NO3 + 4H+ + 3e-→ ClO₂ + e-→ ClO₂- 2Hg²+ + 2e → Hg₂²+ 2+ Ag+ + e → Ag Hg₂+ + 2e → 2Hg Fe³+ + e Fe²+ O₂ + 2H+ + 2e- → H₂O₂ MnO4 + e MnO4²- 1₂ +2e 21- Cute Cu 8° (V) 2.87 1.99 1.82 1.78 1.70 1.69 1.68 1.60 1.51 1.50 1.46 1.36 1.33 1.23 1.21 1.20 1.09 1.00 0.99 0.96 0.954 0.91 0.80 0.80 0.77 0.68 0.56 0.54 0.52 Half-reaction O₂ + 2H₂O + 4e¯→ 40H- Cu²+ + 2e →→ Cu Hg₂Cl₂ + 2e → 2Hg + 2Cl- AgCl+eAg + Cl- SO4 + 4H+ + 2e → H₂SO3 + H₂O Cu²+ + e Cu+ 2H+ + 2e Fe³+ + 3e → H₂ Fe Pb²+ + 2e Sn²+ + 2e Ni²+ + 2e PbSO4 + 2e Cd²+ + 2e Fe2+ + 2e →→ Cd →→ Fe Cr²+ Cr³+ + e- Cr³+ + 3e- → Cr Zn²+ + 2e →→→ Zn 2H₂O + 2e →→ H₂ + 2OH- →→Mn Mn2+ + 2e Al³+ + 3e Al H₂ + 2e → 2H- Mg2+ + 2e → Mg La³+ + 3e La →→ Pb Sn →→→ Ni →→ Pb + SO4²- Nate Na Ca2+ + 2e → Ca Ba2+ + 2e-Ba K+ + e → K Lite Li 8° (V) 0.40 0.34 0.27 0.22 0.20 0.16 0.00 -0.036 -0.13 -0.14 -0.23 -0.35 -0.40 -0.44 -0.50 -0.73 -0.76 -0.83 -1.18 -1.66 -2.23 -2.37 -2.37 2.71 -2.76 -2.90 -2.92 -3.05
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 69% (13 reviews)
The noble metals are relatively difficult to corrode in ai...View the full answer

Answered By

Labindao Antoque
I graduated in 2018 with a Bachelor of Science degree in Psychology from Dalubhasaan ng Lungsod ng San Pablo. I tutored students in classes and out of classes. I use a variety of strategies to tutor students that include: lecture, discussions about the subject matter, problem solving examples using the principles of the subject matter being discussed in class , homework assignments that are directed towards reinforcing what we learn in class , and detailed practice problems help students to master a concept. I also do thorough research on Internet resources or textbooks so that I know what students need to learn in order to master what is being taught in class .
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
In theory, most metals should easily corrode in air. Why? A group of metals called the noble metals are relatively difficult to corrode in air. Some noble metals include gold, platinum, and silver....
-
A group of oligosaccharides called Schardinger dextrins can be isolated from Bacillus macerans when the bacillus is grown on a medium rich in amylose. These oligosaccharides are all nonreducing. A...
-
In 1996, a group of securities called the World Equity Benchmark Shares (WF.BS) started trading on the American Stock Exchange. WEBS for a country is a passively managed ETF indexed on the MSCI...
-
Use the graph of f to solve Exercises 924. Where applicable, use interval notation. Find the x-intercept(s). y = f(x) # [TD y X
-
Lets take a look at Invisible Hand Principle 2 in action using a mathematical example. Suppose an industry is characterized by the equations in the following table. (Were going to assume all...
-
Show that the SUBSET-SUM problem is solvable in polynomial time if the input is given in a unary encoding. That is, show that SUBSET-SUM is not strongly NP-hard. What is the running time of your...
-
Discuss why aligning the development of an SMIS with the organizations strategy is important. Describe the process of developing an effective SMIS. List four common social media goals and describe...
-
On January 1, 2009, Plymouth Corporation acquired 80 percent of the outstanding voting stock of Sander Company in exchange for $1,200,000 cash. At that time, although Sanders book value was $925,000,...
-
Describe the scope and objectives of audit work and identify the major steps in the audit process?
-
A simple security system for two doors consists of a card reader and a keypad. A person may open a particular door if he or she has a card containing the corresponding code and enters an authorized...
-
Consider a galvanic cell based on the following halfreactions: a. What is the expected cell potential with all components in their standard states? b. What is the oxidizing agent in the overall cell...
-
Electrolysis of an alkaline earth metal chloride using a current of 5.00 A for 748 seconds deposits 0.471 g of metal at the cathode. What is the identity of the alkaline earth metal chloride?
-
Suppose you heard that an investment bank decided not to form a syndicate to underwrite an issue of securities. What does this piece of information tell you about the quality of the securities soon...
-
Part 1 of 4 05 points abook Print References Required information Problem 24-2A (Algo) Payback period, accounting rate of return, net present value, and net cash flow calculation LO P1, P2, P3 [The...
-
Keenan Music's CEO has been pondering about the recent proposal of the Specialty Guitar Project. The accountant has done a capital budgeting analysis on the project and outlined the conditions that...
-
On July 1, 2025, Sheridan Co. pays $15,000 to Blue Spruce Insurance Co. for a 2-year insurance contract. Both companies have fiscal years ending December 31. (a1) Journalize the entry on July 1 and...
-
A CU triaxial test with c = 20 psi is performed on a sand and a deviator stress of 80 psi fails the specimen. Previous tests revealed that the effective friction angle for this sand is 35. Calculate...
-
Haliburton Mills Inc. is a large producer of men's and women's clothing. The company uses standard costs for all of its products. The standard costs and actual costs for a recent period are given...
-
Which figure in this chapter best shows that a constellation seen in the background of a solar eclipse is one that will be seen 6 months later in the night sky?
-
The power company must generate 100 kW in order to supply an industrial load with 94 kW through a transmission line with 0.09 resistance. If the load power factor is 0.83 lagging, find the...
-
Which of the following sets of quantum numbers are not allowed? For each incorrect set, state why it is incorrect. a. n = 3, = 3, m = 0, ms = - 1/2 b. n = 4, = 3, m = 2, ms = - 1/2 c. n = 4, = 1,...
-
How many orbitals can have the designation 5p, 3dz2, 4d, n = 5, and n = 4?
-
How many electrons in an atom can have the designation 1p, 6dx2-y2, 4f, 7py, 2s, and n = 3?
-
What is the present value of $500 invested each year for 10 years at a rate of 5%?
-
GL1203 - Based on Problem 12-6A Golden Company LO P2, P3 Golden Corp.'s current year income statement, comparative balance sheets, and additional information follow. For the year, (1) all sales are...
-
A project with an initial cost of $27,950 is expected to generate cash flows of $6,800, $8,900, $9,200, $8,100, and $7,600 over each of the next five years, respectively. What is the project's...

Study smarter with the SolutionInn App


