In which of the following systems is(are) work done by the surroundings on the system? Assume pressure
Question:
In which of the following systems is(are) work done by the surroundings on the system? Assume pressure and temperature are constant.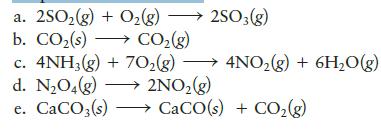
Transcribed Image Text:
a. 2SO₂(g) + O₂(g) →→→ 2SO3(g) b. CO₂ (s) CO₂(g) → c. 4NH3(g) + 70₂(g) d. N₂O4(g) →→→ 2NO₂(g) e. CaCO3(s)→→→→→→ CaCO(s) + CO₂(g) 4NO₂(g) + 6H₂O(g) →
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (12 reviews)
e CaCO3s CaCOs CO2g In this system work is done by ...View the full answer

Answered By

Sylvanus Oyoo
a business student specifically specialized in the field of accounting
also an IT expert
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
In which of the following systems is the energy level separation the largest? (a) A 14Nnucleus in (for protons) a 600 MHz NMR spectrometer, (b) An electron in a radical in a field of 0.300 T
-
Which of the following systems has a unique solution? (a) (b) (c) (d) (e) 022 110 2 xyz 0 3 2 132 130 202 211 132 433 112 0 pqrs 002 250 491
-
Which of the following systems has (i) A unique solution? (ii) Infinitely many solutions? (iii) No solution? In each case, find all solutions: (a) x-2y = 1 3x + 2y = -3 (b) 2x + y + 3z = l x + 4y -...
-
1. As shown by point D in Fig 3.1, the volume of an ideal diatomic gas is 2.00L at standard condition (STP, T=273.15K, P=101.3kPa). The gas is heated to A with its volume conserved, expands...
-
Suppose that a random sample of n observations is drawn from a distribution for which the p.d.f. is as given in Exercise 14. Determine the asymptotic distribution of the sample median.
-
Lancer Audio produces a high-end 7.2 channel AV receiver that sells for $1,300. Total operating expenses for July were as follows: Units produced and sold........................150 Component...
-
2.1 A field experiment to investigate the effect of four levels of nitrogen fertilizer on the yield of three varieties of oats was laid out in a split plot design (Yates, 1937). The experiment...
-
Compare briefly the major types of employment interviews described in this chapter. Which type would you prefer to conduct? Why?
-
A company takes out a loan. The loan is $10000 and has an annual effetive interest rate of 5.82%. you are given (a) the loan is to be repaid with no annual payments of $1200 per year plus a payment k...
-
A school administrator is interested in finding how the threatened teachers strike can be averted. He knows that pay demands and the classrooms physical environment are the two main issues in the...
-
Which of the following processes are exothermic? a. N(g) 2N(g) b. HO(l) HO(s) c. Cl(g) 2Cl(g) d. 2H(g) + O(g) 2HO(g) e. O(g) 20(g)
-
Nitromethane, CH3NO2, can be used as a fuel. When the liquid is burned, the (unbalanced) reaction is mainly a. The standard enthalpy change of reaction (DH 8 rxn) for the balanced reaction (with...
-
The post-closing trial balance may contain revenue and expense accounts.
-
1. Define a person-centered model of care in LTC facilities. 2. Describe two leadership behaviors and two leadership qualities most conducive to moving long-term care organizations toward more...
-
question 5 all parts 8+0.5 = 4. Consider a system with a lead compensator Ge(s) = +0.13 followed by a plant G(s) = 10 Determine a value for a gain K on the error signal such that the phase margin...
-
3- Define and describe, in detail, the various communication styles as they relate to negotiation and conflict resolution. Compare the advantages and disadvantages of the styles. Provide a detailed...
-
SJ Corp ahs the following data for 2020: RM, beginning of 5,000; Purchases of raw materials is 50,000; return of defective raw materials to suppliers of 4,000; return of direct materials from the...
-
A company is issuing $340,000 worth of 4-year bonds on October 8, 2023, bearing an interest rate of 2%, payable annually. Assume that the current market rate of interest is 3%. a) Will the bonds be...
-
Consider a wave that has a frequency of 3.0 kHz and a wavelength of 0.10 m. Write an equation that might describe this wave.
-
A consultant is beginning work on three projects. The expected profits from these projects are $50,000, $72,000, and $40,000. The associated standard deviations are $10,000, $12,000, and $9,000....
-
A galvanic cell is based on the following half reactions: Ag+ + e2 Ag(s) o = 0.80 V Cu2+ + 2e2 Cu(s) o = 0.34 V In this cell the silver compartment contains a silver electrode and excess AgCl(s)...
-
Consider the following galvanic cell: Calculate the Ksp value for Ag2SO4(s). Note that to obtain silver ions in the right compartment (the cathode compartment), excess solid Ag2SO4 was added and some...
-
Consider the following galvanic cell: Ag Cd 1.00 M Ag+ 1.00M Cd2+
-
Selected comparative financial statement data for DAS inc. Balance Sheet (En milliers de dollars) 2017 2018 Assets Assets CT - Cash 41.63 47.5 - Accounts Receivable 64.2 72.6 - inventories 969.7...
-
please help!! One chance at turning in!!! 16 rows! I'd highly appreicate it I am unsure what information you need... I provided all Current Attempt in Progress Mike Greenberg opened Grouper Window...
-
Blue Ridge Marketing Inc. manufactures two products, A and B . Presently, the company uses a single plantwide factory overhead rate for allocating overhead to products. However, management is...

Study smarter with the SolutionInn App


