Name the amino acids in Table 11E.3 that contain nonpolar side groups. These groups contribute to the
Question:
Name the amino acids in Table 11E.3 that contain nonpolar side groups. These groups contribute to the tertiary structure of a protein by preventing contact with water.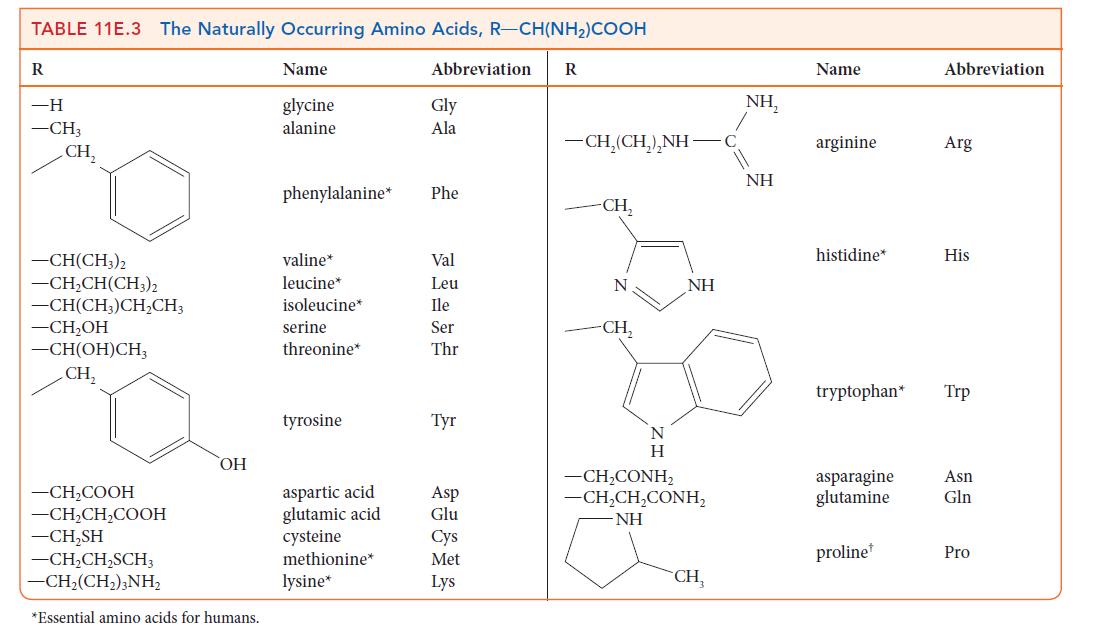
Transcribed Image Text:
TABLE 11E.3 The Naturally Occurring Amino Acids, R-CH(NH)COOH R -H -CH3 CH -CH(CH3)2 -CHCH(CH3)2 -CH(CH3)CHCH3 -CHOH -CH(OH)CH CH, -CHCOOH -CHCHCOOH -CHSH -CHCHSCH3 CH,(CH,),NH, OH *Essential amino acids for humans. Name glycine alanine phenylalanine* valine* leucine* isoleucine* serine threonine* tyrosine aspartic acid glutamic acid cysteine methionine* lysine* Abbreviation Gly Ala Phe Val Leu Ile Ser Thr Tyr Asp Glu Cys Met Lys R CH,(CH,),NH -CH N -CH N H NH CH,CONH, CH,CH,CONH, NH CH NH, NH Name arginine histidine* tryptophan* asparagine glutamine proline Abbreviation Arg His Trp Asn Gln Pro
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
From the image provided Table 11E3 lists the naturally occurring amino acids their structures names ...View the full answer

Answered By

Loise Ndungu
I have five years of experience as a writer. As I embark on writing your papers from the prologue to the epilogue, my enthusiasm is driven by the importance of producing a quality product. I put premium product delivery as my top priority, as this is what my clients are seeking and what makes me different from other writers. My goal is to craft a masterpiece each time I embark on a freelance work task! I'm a freelance writer who provides his customers with outstanding and remarkable custom writings on various subjects. Let's work together for perfect grades.
4.90+
82+ Reviews
236+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Name the amino acids in Table 11E.3 that contain side groups capable of forming hydrogen bonds. This interaction contributes to the tertiary structure of a protein. TABLE 11E.3 The Naturally...
-
Which amino acids in Table 17.1 have nonpolar R groups? Highly polar groups? Relatively flat R groups? Table 17.1 QUESTION CONTINUE TO NEXT PAGE Table 17.1 Names and Formulas of the Common Amino...
-
Let {Wt} be an SBM and define the process {Bt} by Bt = Wt t*W10/10, t [0, 10]. (a) For t [0, 10], what is the probability distribution of Bt? (b) Fix t [0, 10] and consider the bivariate random...
-
Accounting The Case: Patient Khaled is a 75-year-old man admitted to the hospital for a small bowel obstruction. His medical history includes hypertension. Khaled is on NPO. He has a nasogastric (NG)...
-
Use source transformation to determine Io in the network infigure. V2 4/0 A -j1 n 12/0 V 2/00 A
-
One of the biggest problems of student writers is paraphrasing secondary sources correctly to avoid plagiarism. Your Task. For each of the following, read the original passage. Analyze the...
-
5. After the 10 minutes have ended, determine how many yachts each group made and assess the quality of the work. As a class, discuss the groups performance. Did each group meet its supervisors...
-
The intangible assets reported by Ip Company at December 31, 2016, follow: Patent #1 was acquired in January 2015 and has an estimated useful life of 10 years. Copyright #1 was acquired in January...
-
The December 3 1 , 2 0 2 4 , adjusted trial balance for the Blueboy Cheese Corporation is presented below. \ table [ [ Account Title,Debits,Credits ] , [ Cash , $ 1 7 , 3 0 0 , ] , [ Accounts...
-
Give the systematic name of each of the following amines: (a) CH 3 NH 2 ; (b) (CH 3 CH 2 ) 2 NH; (c) o-CH 3 C 6 H 4 NH 2 .
-
(a) How many liters of hydrogen at 1.00 atm and 298 K are needed to hydrogenate (i) 1.00 mol C 6 H 10 , cyclohexene; (ii) 1.00 mol C 6 H 6 , benzene, completely? (b) Estimate the reaction enthalpy of...
-
Referring to Exercise 9.23, construct a 95% confidence interval for the population standard deviation of the diameters of Indian mounds.
-
You have been employed as a systems analyst in the information systems organization of a medium-sized consumer goods manufacturer for three years. You are quite surprised when your manager offers you...
-
For your initial post, address the following: First, introduce yourself to the class by sharing a bit about yourself, such as your preferred name or pronouns, where you are from, what your major is,...
-
Question 8 : Consider the technology of Solar Panels. Which stage of the technology life cycle S curve is this technology in. Justify why ? Question 9 : The standard Product Life Cycle has 5 stages...
-
At Benihana restaurant a man wrenched his neck while ducking a piece of flying shrimp, requiring treatment by several doctors. By that summer, doctors determined surgery was necessary to treat...
-
You have just come into an inheritance of $25,000 from a distant relative, and you want to invest it for the long term. Provide an investment portfolio that includes five different stocks. Report the...
-
Olympic athletes are tested to see if they are using illegal performance-enhancing drugs. Suppose that urine samples are taken and analyzed and the rate of false positive results is 1%. Suppose also...
-
Use the information given about the angles and to find the exact value of: (a) sin( + ) (b) cos( + ) (c) sin( - ) (d) tan ( + ) (e) sin(2) (f) cos (2) (g) sin /2 (h) cos/2 cos = 4/5, 0 < < /2; cos =...
-
Each of the following aldehydes was converted into an -amino nitrile followed by hydrolysis to yield an amino acid. In each case, draw and name the amino acid that was produced. (a) Acetaldehyde (b)...
-
Identify the reagents necessary to make each of the following amino acids using a HellVolhardZelinski reaction. (a) Leucine (b) Alanine (c) Valine
-
Draw the aldehyde that is obtained as a byproduct when l-leucine is treated with ninhydrin.
-
Your firm is planning to invest in an automated packaging plant. Harburtin Industries is an all - equity firm that specializes in this business. Suppose Harburtin ' s equity beta is 0 . 8 7 , the...
-
Ned Allen opened a medical practice in Los Angeles, California, and had the following transactions during the month of January. (Click the icon to view the January transactions.) Journalize the...
-
do you need more information or are you working on this? Irene Watts and John Lyon are forming a partnership to which Watts will devote one- half time and Lyon will devote full time. They have...

Study smarter with the SolutionInn App


