Suppose that 15.0 mL of 0.15 m NH 3 (aq) is titrated with 0.10 m HCl(aq). (a)
Question:
Suppose that 15.0 mL of 0.15 m NH3(aq) is titrated with 0.10 m HCl(aq).
(a) What is the initial pH of the 0.15 m NH3(aq)?
(b) What is the pH after the addition of 15.0 mL of 0.10 m HCl(aq)?
(c) What volume of 0.10 m HCl(aq) is required to reach halfway to the stoichiometric point?
(d) Calculate the pH at the halfway point.
(e) What volume of 0.10 m HCl(aq) is required to reach the stoichiometric point?
(f) Calculate the pH at the stoichiometric point.
(g) Use Table 6H.2 to select an indicator for the titration.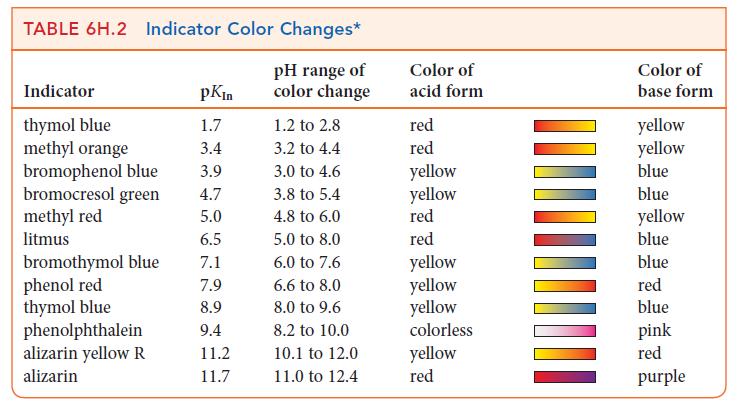
Transcribed Image Text:
TABLE 6H.2 Indicator Color Changes* pH range of color change Indicator thymol blue methyl orange bromophenol blue bromocresol green methyl red litmus bromothymol blue phenol red thymol blue phenolphthalein alizarin yellow R alizarin pKin 1.7 3.4 3.9 4.7 5.0 6.5 7.1 7.9 8.9 9.4 11.2 11.7 1.2 to 2.8 3.2 to 4.4 3.0 to 4.6 3.8 to 5.4 4.8 to 6.0 5.0 to 8.0 6.0 to 7.6 6.6 to 8.0 8.0 to 9.6 8.2 to 10.0 10.1 to 12.0 11.0 to 12.4 Color of acid form red red yellow yellow red red yellow yellow yellow colorless yellow red Color of base form yellow yellow blue blue yellow blue blue red blue pink red purple
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (5 reviews)
a Initial pH 1120 b pH ...View the full answer

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Suppose that 50.0 mL of 0.25 m CH 3 NH 2 (aq) is titrated with 0.35 m HCl(aq). (a) What is the initial pH of the 0.25 m CH 3 NH 2 (aq)? (b) What is the pH after the addition of 15.0 mL of 0.35 m...
-
Please write detailed roadmap/solution for all questions below. 1) An output of nmap search is shown below, a) Type the required terminal command and required parameters to obtain the shown output....
-
Below is the titration curve for the neutralization of 25 mL of a monoprotic acid with a strong base. Answer the following questions about the reaction and explain your reasoning in each case. (a) Is...
-
Northland Corporation is a small information-systems consulting firm that specializes in helping companies implement standard sales-management software. The market for Northalndss services is very...
-
The driver of a car traveling at 100 km/h toward a vertical cliff briefly sounds the horn. Exactly one second later she hears the echo and notes that its frequency is 840 Hz. How far from the cliff...
-
Suppose rRF = 9%, rM = 14%, and bi = 1.3. a. What is ri, the required rate of return on Stock i? b. Now suppose rRF (1) increases to 10 percent or (2) decreases to 8 percent. The slope of the SML...
-
7. Suppose that V is open in Rn , f: V -+ R is C2 on V, and fxJa) = for some a E V and all j = 1, ... , n. Prove that if H is a compact convex subset of V, then there is a constant M such that for...
-
Weller Company's flexible budget for manufacturing overhead (in condensed form) follows The following information is available for a recent period: a. The denominator activity of 8,000 machine-hours...
-
Star clusters are ideal natural laboratories to study stars and our models of stellar evolution. Higher mass stars leave the main sequence faster than lower mass stars. For a star cluster, where all...
-
For the partially submerged backfill in Problem 13.13 (Figure 13.37), determine the Rankine's passive force per unit length of the wall and the location of the resultant In problem 13 Problem HH Y1 ...
-
A metal alloy sample is believed to contain silver, bismuth, and nickel. Explain how it could be determined qualitatively that all three of these metals are present.
-
Calculate the pH of (a) 0.15 m CH 3 NH 3 Cl(aq); (b) 0.063 m FeCl 3 (aq).
-
Explain why vinyl shower curtains develop cracks over time.
-
You are the cost accountant of an engineering concern which has three departments - preparation, machining and assembly. The budgeted direct labour hours for the workshops are 8,000, 12,000 and...
-
What alternative to fostering fun and enjoyment at work do you think might have worked for Zappos?
-
Using the techniques of dimensional analysis, and assuming that experimentation shows the dimensionless number to be 1, derive the following equation: E v = Job card two The results of an ultrasonic...
-
Given the historical cost of product Carla Vista is $13, the selling price of product Carla Vista is $15, costs to sell product Carla Vista are $3, the replacement cost for product Carla Vista is...
-
What causes of outliers in statistics and when I create a boxplot why do I not see the outliers. What steps are to take in creating a boxplot?
-
Consider a memory system that uses a 32-bit address to address at the byte level, plus a cache that uses a 64-byte line size. a. Assume a direct mapped cache with a tag field in the address of 20...
-
Akramin just graduated with a Master of Engineering in Manufacturing Engineering and landed a new job in Melaka with a starting salary of RM 4,000 per month. There are a number of things that he...
-
Can a gas be liquefied through an isenthalpic expansion if J T = 0?
-
Arrange the following compounds in order of increasing boiling point: -, - -, -0
-
Why is q V = U only for a constant volume process? Is this formula valid if work other than P V work is possible?
-
Columbus Industries makes a product that sells for $37 a unit. The product has a $29 per unit variable cost and total fixed costs of $10,000. At budgeted sales of 1,950 units, the margin of safety...
-
18. Suppose that Maxima shares are selling for $10 per share and you own a call option to buy Maxima shares at $7.50. The intrinsic value of your option is:
-
ABC Insurance Company reported the following information on its accounting statements last year: What was ABC 's expense ratio last year

Study smarter with the SolutionInn App


